Osmotic pressure is an important factor that affects cells. Osmosis is the net movement of solvent molecules through a partially permeable membrane into a region of higher solute concentration. The intent of osmosis is to equalize the solute concentrations on the two sides. Osmosis is essential in biological systems because biological membranes are semi permeable. In general, these membranes are impermeable to large and polar molecules such as ions, proteins, and polysaccharides. However, they are permeable to non-polar and/or hydrophobic molecules like lipids as well as to small molecules like oxygen, carbon dioxide, nitrogen, nitric oxide, etc. Osmosis provides the primary means by which water is transported into and out of cells. Osmoregulation is the homeostasis mechanism of an organism to reach balance in osmotic pressure.
Having the correct osmotic pressure in the culture medium is essential. A cell can be influenced by a solution in three ways. Suppose a cell is placed in a solution of sugar or salt water. If the medium is hypotonic — a diluted solution with a higher water concentration than the cell — the cell will gain water through osmosis . If the medium is isotonic — a solution with exactly the same water concentration as the cell — there will be no net movement of water across the cell membrane . If the medium is hypertonic — a concentrated solution with a lower water concentration than the cell — the cell will lose water by osmosis .
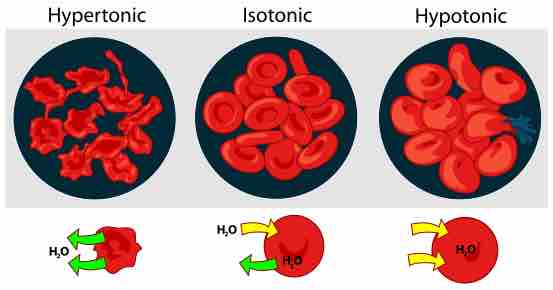
Osmotic Pressure on Red Blood Cells
Effect of different solutions on blood cells.
Essentially, this means that if a cell is put in a solution that has a solute concentration higher than its own, then it will shrivel up. If it is put in a solution with a lower solute concentration than its own, the cell will expand and burst.