Coordination Complexes in Biology
Coordination complexes (also called coordination compounds) and transition metals are widespread in nature. They are an integral component of proteins, especially the class of proteins that can perform chemical reactions, called enzymes. The transition metals, particularly zinc and iron, are often key components of enzyme active sites. While there are other biologically relevant molecules that also contain metals, coordination complexes contain a central metal ion and are important in many biological processes.
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large fraction of all proteins are metalloenzymes, and they have many diverse functions including transport, storage, and signal transduction.
Coordination Chemistry Principles
In metalloproteins, metal ions are usually coordinated by nitrogen, oxygen, or sulfur centers belonging to amino acid residues of the protein. These donor groups are often provided by side-chains on the amino acid residues. Important donor groups include:
- imidazole (a nitrogen atom donor) substituents in histidine residues
- thiolate (sulfur atom) substituents in cysteinyl residues
- carboxylate groups (oxygen atom) provided by aspartate
Given the diversity of metalloproteins, virtually all amino acid residues have been shown to bind metal centers. The peptide backbone also provides donor groups; these include deprotonated amides and the amide carbonyl oxygen centers (oxygen and nitrogen atoms as ligands).
In addition to donor groups that are provided by amino acid residues, a large number of organic cofactors function as ligands. Perhaps most famous are the tetradentate N4 macrocyclic ligands incorporated into the heme protein (most commonly seen as part of hemoglobin). Inorganic ligands such as sulfide and oxide are also common.
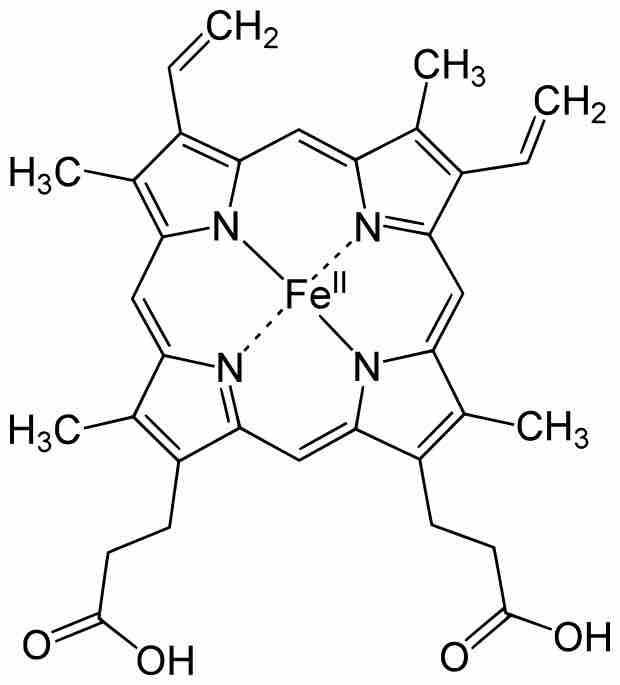
Heme B
Heme B is a porphyrin (four linked pyrrole rings) that readily binds iron, as shown. This is an example of a biomolecule that contains non-protein ligands for a transition metal.
Metalloenzymes
Metalloenzymes contain a metal ion bound to the protein with one labile coordination site. As with all enzymes, the shape of the active site is crucial. The metal ion is usually located in a pocket whose shape fits the substrate. The metal ion catalyzes reactions that are difficult to achieve in organic chemistry. Consider the following reaction:
This reaction is very slow in the absence of a catalyst, but quite fast in the presence of the hydroxide ion.
A reaction similar to this is almost instantaneous with carbonic anhydrase. The structure of the active site in carbonic anhydrases is well known from a number of crystal structures. It consists of a zinc ion coordinated by three imidazole nitrogen atoms from three histidine units. The fourth coordination site is occupied by a water molecule.
The positively charged zinc ion polarizes the coordinated water molecule, and the nucleophilic attack by the negatively charged portion on carbon dioxide proceeds rapidly. The catalytic cycle produces the bicarbonate ion and the hydrogen ion as the equilibrium favors dissociation of carbonic acid at biological pH values.
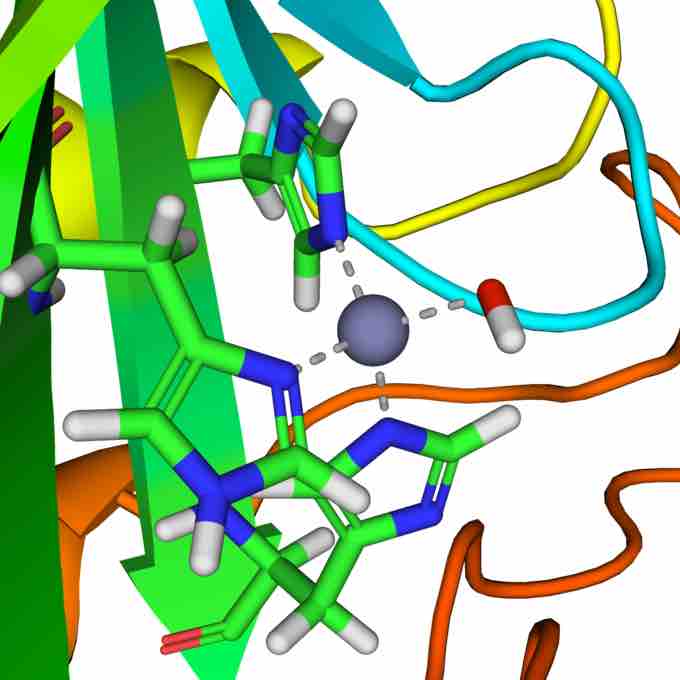
Carbonic anhydrase active site
Active site of carbonic anhydrase. The three coordinating histidine residues are shown in green, hydroxide in red and white, and the zinc in gray.