Hydrophilic Colloids
A hydrophilic colloid, or hydrocolloid, is defined as a colloid system in which the colloid particles are hydrophilic polymers dispersed in water. Hydrocolloids can be either reversible or irreversible (single-state). For example, agar is a reversible hydrocolloid of seaweed extract; it can exist in a gel or liquid state and can alternate between states with either heating or cooling.
Agar plate
Petri dishes containing agar jelly for bacterial culture
Many hydrocolloids are derived from natural sources. For example, gelatin is produced by hydrolysis of proteins from cows and fish, and pectin is extracted from citrus peel and apple pomace. Hydrocolloid-based medical dressings are used for skin and wound treatment.
Hydrophobic Colloids
A hydrophobic colloid, or emulsion, is defined as a colloid system where the particles are hydrophobic polymers. Since the colloid does not interact with the aqueous solvent, hydrophobic colloids are inherently unstable and generally do not form spontaneously. Energy input, through shaking, stirring, or homogenizing, is needed to form the emulsion. Over time, an emulsion tends to separate, because separation puts it in a more stable state. An example of this is seen in the separation of the oil and vinegar components of vinaigrette, an unstable emulsion that will quickly separate unless shaken almost continuously.
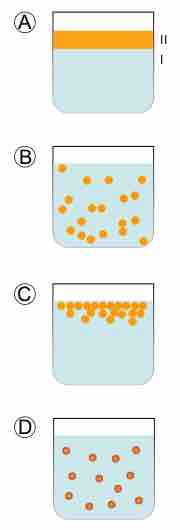
Hydrophobic colloid stages
In A, there are two immiscible liquids that are not yet emulsified. In B, an emulsion of Phase II dispersed in Phase I has been formed. In C, the unstable emulsion progressively separates. In D, an emulsifier (purple outline around particles) positions itself on the interface between Phase II and Phase I to stabilize the emulsion.
There are three types of instability in emulsions: flocculation, creaming, and coalescence. In flocculation, the dispersed phase comes out of suspension in the form of flakes. In creaming, one of the substances migrates to the top or bottom, depending on the relative densities involved. In coalescence, small droplets of colloid bump into each other and combine to form progressively larger droplets. In order for the emulsion to stay stable, additional substances are needed to stabilize the colloid.
An emulsifier is a substance that stabilizes the colloid so that it does not change significantly with time. One important class of emulsifiers is known as "surface active substances," or surfactants. Soy and egg yolk lecithin are examples of surfactants. Surfactants are often used in food, such as mayonnaise and salad dressing, to keep the emulsions mixed over time. Detergents are another class of surfactants, and they will physically interact with both oil and water, thus stabilizing the interface between the oil and water droplets in suspension. This principle is also exploited in soap, to remove grease for the purpose of cleaning.