The noble gases are a group of chemical elements that make up Group 18 on the periodic table. These gases all have similar properties under standard conditions: they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six noble gases that occur naturally are helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn).
Properties of the Noble Gases
The properties of the noble gases can be well explained by modern theories of atomic structure. The outer shell of valence electrons is considered to be "full" in noble gases, giving them little tendency to participate in chemical reactions. It has been possible to prepare only a few hundred noble gas compounds. In the case of Neon (Ne), for example, both the n = 1 and n = 2 shells are complete and therefore it is a stable monatomic gas under ambient conditions.
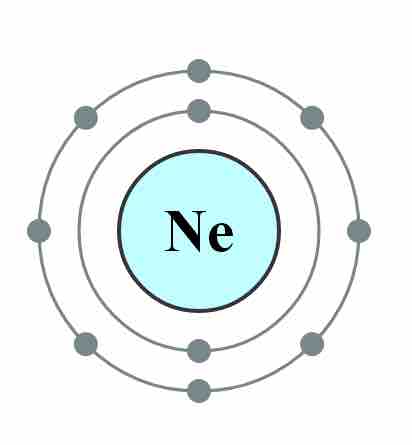
Electron configuration of neon
The electron configuration of Neon (Ne), with two complete energy levels, 1s2 and 2s2 2p6.
The melting and boiling points (physical properties) of a noble gas are close together, differing by less than 10 °C (18 °F) – that is to say, they are liquids over only a small temperature range.
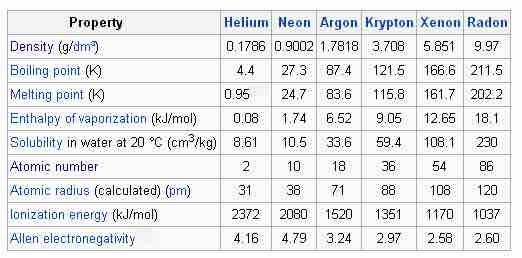
Physical properties of the noble gases
The physical properties of the noble gases are tightly grouped.
The noble gases have weak interatomic forces and consequently have very low melting and boiling points. They are all monatomic gases under standard conditions, including those with larger atomic masses than many other elements that are solids under standard conditions.
Electron Configurations in the Noble Gases
The noble gas atoms, as do the atoms in most other groups on the periodic table, increase steadily in atomic radius from one period to the next due to an increasing number of electrons. The size of the atom is related to several properties. For example, the ionization potential decreases with an increasing radius because the valence electrons in the larger noble gases are farther away from the nucleus and so are not held as tightly together by the atom. Noble gases have the largest ionization potential among the elements of each period. This reflects the stability of their electron configuration and points again to their relative lack of chemical reactivity.
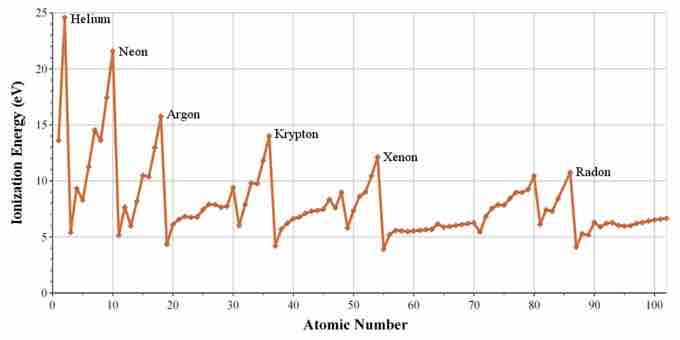
Ionization potential for noble gases
The noble gases have the largest ionization potential for the elements in their respective periods.
Inert Gases
The noble gases were originally also referred to as "inert gases," since it was believed that they did not react with other elements to form compounds. In recent years, however, this term has fallen out of favor, although you will occasionally see it in older literature. Scientists have discovered that, since the heavier noble gas atoms are held together less strongly by electromagnetic forces than are the lighter noble gases, such as helium, the outer electrons of these heavier atoms can be removed more easily. Because of this, many compounds of the gases xenon, krypton, and radon can, in fact, be formed. Of the six noble gases, only krypton, xenon, and radon have the ability to form stable compounds. These are used as oxidizing agents.
Applications for the Noble Gases
The noble gases glow in distinctive colors when used inside gas-discharge lamps, such as neon lights. Xenon is commonly used in xenon arc lamps, which are present in film projectors and automobile headlights due to their nearly continuous spectrum that resembles daylight.
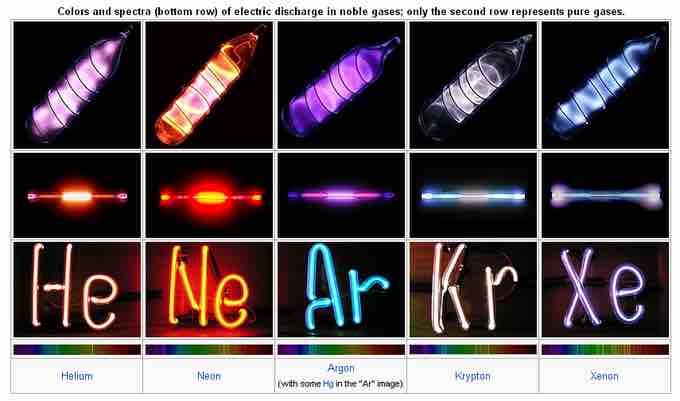
A very common use of the noble gases
Under the correct conditions, brightly lit and colorful signs can be made using noble gases. "Neon Lights" is the common term, but any of the noble gases can be used.
The noble gases are also used in excimer lasers, which are based on short-lived electronically excited molecules known as excimers. The excimers used for lasers may be noble gas dimers such as Ar2, Kr2, or Xe2, or, more commonly, the noble gas is combined with a halogen in excimers such as ArF, KrF, XeF, or XeCl. These lasers produce ultraviolet light, which, due to its short wavelength (193 nm for ArF and 248 nm for KrF), allows for high-precision imaging. Excimer lasers have many industrial, medical, and scientific applications. They are used for microlithography and microfabrication, which are essential for integrated circuit manufacturing; and for laser surgery, including laser angioplasty and eye surgery.