Periodic Trends in the Ionization Energy
The ionization energy of a chemical species (i.e., an atom or molecule) is the energy required to remove electrons from gaseous atoms or ions. This property is also referred to as the ionization potentia and is measured in volts. In chemistry, it often refers to one mole of a substance (molar ionization energy or enthalpy) and is reported in kJ/mol. In atomic physics, the ionization energy is typically measured in the unit electron volt (eV). Large atoms or molecules have low ionization energy, while small molecules tend to have higher ionization energies.
The ionization energy is different for electrons of different atomic or molecular orbitals. More generally, the nth ionization energy is the energy required to strip off the nth electron after the first n-1 electrons have been removed. It is considered a measure of the tendency of an atom or ion to surrender an electron or the strength of the electron binding. The greater the ionization energy, the more difficult it is to remove an electron. The ionization energy may be an indicator of the reactivity of an element. Elements with a low ionization energy tend to be reducing agents and form cations, which in turn combine with anions to form salts.
Ionization energy
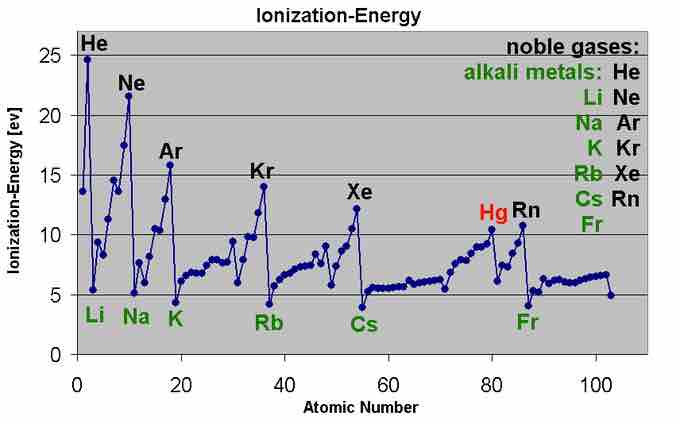
This graph shows the first ionization energy of the elements in electron volts.
Moving left to right within a period or upward within a group, the first ionization energy generally increases. As the atomic radius decreases, it becomes harder to remove an electron that is closer to a more positively charged nucleus. Conversely, as one progresses down a group on the periodic table, the ionization energy will likely decrease since the valence electrons are farther away from the nucleus and experience greater shielding. They experience a weaker attraction to the positive charge of the nucleus. Ionization energy increases from left to right in a period and decreases from top to bottom in a group.
Rationale for the Periodic Trends in Ionization Energy
The ionization energy of an element increases as one moves across a period in the periodic table because the electrons are held tighter by the higher effective nuclear charge. This is because additional electrons in the same shell do not substantially contribute to shielding each other from the nucleus, however an increase in atomic number corresponds to an increase in the number of protons in the nucleus.
The ionization energy of the elements increases as one moves up a given group because the electrons are held in lower-energy orbitals, closer to the nucleus and thus more tightly bound (harder to remove).
Based on these two principles, the easiest element to ionize is francium and the hardest to ionize is helium.