Blocks of the Periodic Table
The periodic table does more than just list the elements. The word "periodic" means that within each row, or period, the elements show a pattern of characteristics. This is because the elements are listed in part by their electron configuration.
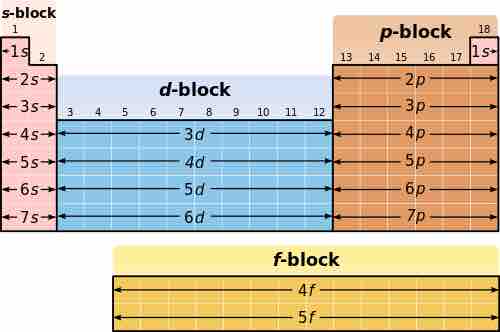
Blocking in the periodic table
The periodic table can be broken into blocks, corresponding to the highest energy electrons.
The alkali metals and alkaline earth metals have one and two valence electrons (electrons in the outer shell), respectively; because of this, they lose electrons to form bonds easily and so are very reactive. These elements comprise the s block of the periodic table. The p block, on the right, contains common non-metals, such as chlorine and helium. The noble gases, in the column on the right, almost never react, since they have eight valence electrons forming a stable outer shell. The halogens, directly to the left of the noble gases, readily gain electrons and react with metals. The s and p blocks make up the main-group elements, also known as representative elements. The d block, which is the largest, consists of transition metals, such as copper, iron, and gold. The f block, on the bottom, contains rarer metals, including uranium. Elements in the same group or family have the same configuration of valence electrons, so they behave in chemically similar ways.
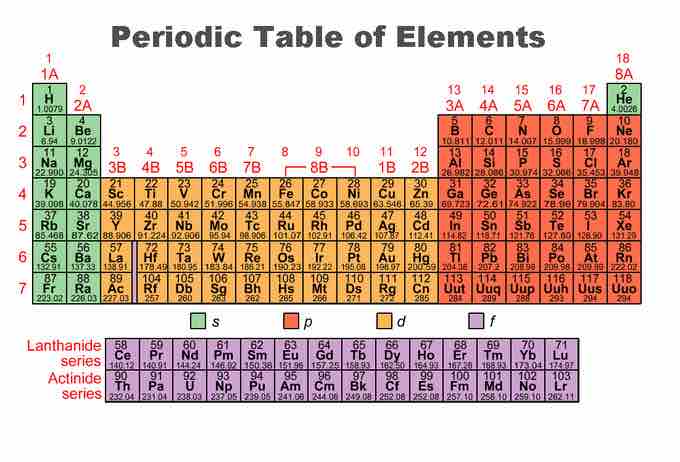
Periodic table of the elements
This image is color-coded to show the s, p, d, and f blocks of the periodic table.
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. For example, the electron configuration of the neon atom (Ne) is 1s2 2s2 2p6. According to the laws of quantum mechanics, a certain energy is associated with each electron configuration. Under certain conditions, electrons can move from one orbital to another by emission or absorption of a quantum of energy, in the form of a photon.
Knowledge of the electron configurations of different atoms is useful in understanding the structure of the periodic table. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.
The idea of an electron configuration was first conceptualized under the Bohr model of the atom, and it is still common to speak of "shells" and "subshells" despite the advances in understanding of the quantum-mechanical nature of electrons.
Aufbau Principle
The Aufbau principle (from the German Aufbau, meaning "building up, construction;" also called the Aufbau rule or building-up principle) is used to determine the electron configuration of an atom, molecule, or ion. The principle postulates a hypothetical process in which an atom is "built up" by the progressive addition of electrons. As electrons are added, they assume their most stable positions (electron orbitals) with respect to the nucleus and the electrons that are already there.
According to the principle, electrons fill orbitals starting at the lowest available energy state before filling higher states (e.g., 1s before 2s). The number of electrons that can occupy each orbital is limited by the Pauli exclusion principle. If multiple orbitals of the same energy are available, Hund's rule states that unoccupied orbitals will be filled before occupied orbitals are reused (by electrons having different spins).

Atomic orbitals ordered by increasing energy
Order in which orbitals are arranged by increasing energy according to the Madelung rule. Each diagonal red arrow corresponds to a different value of n + l.
Magnetism
Magnetism is a property of materials that respond to an applied magnetic field. Permanent magnets have persistent magnetic fields caused by ferromagnetism, the strongest and most familiar type of magnetism. However, all materials are influenced differently by the presence of a magnetic field. Some are attracted to a magnetic field (paramagnetism); others are repulsed by it (diamagnetism); still others have a much more complex relationship with an applied magnetic field (e.g., spin-glass behavior and antiferromagnetism). Substances that are negligibly affected by magnetic fields are considered non-magnetic, these are: copper, aluminum, gases, and plastic. Pure oxygen exhibits magnetic properties when cooled to a liquid state.
The magnetic properties of a given element depend on the electron configuration of that element, which will change when the element loses or gains an electron to form an ion. If the ionization of an element yields an ion with unpaired electrons, these electrons may align the sign of their spins in the presence of a magnetic field, making the material paramagnetic. If the spins tend to align spontaneously in the absence of a magnetic field, the resulting species is termed ferromagnetic.
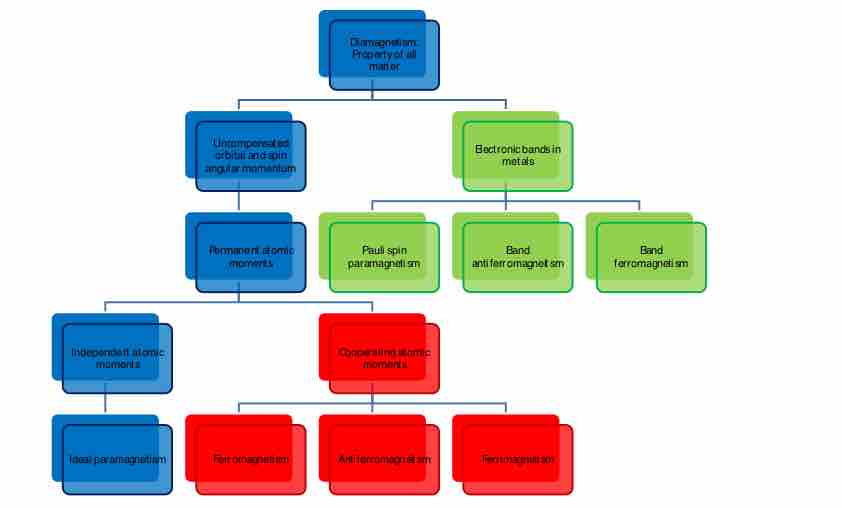
Hierarchy for various types of magnetism
There are various types of magnetism identified to date that can be organized in a hierarchy.
Applications of Magnetism
A lodestone, or loadstone, is a naturally magnetized piece of the mineral magnetite (Fe3O4). Ancient people first discovered the property of magnetism in lodestone. Pieces of lodestone, suspended so they could turn, were the first magnetic compasses, and their importance to early navigation is indicated by their very name, which in Middle English means "course stone" or "leading stone." Lodestone is one of only two minerals that is found naturally magnetized; the other, pyrrhotite, is only weakly magnetic.