In chemistry, periodic trends are the tendencies of certain elemental characteristics to increase or decrease as one progresses along a row or column of the periodic table of elements. The atomic radius is one such characteristic that trends across a period and down a group of the periodic table.
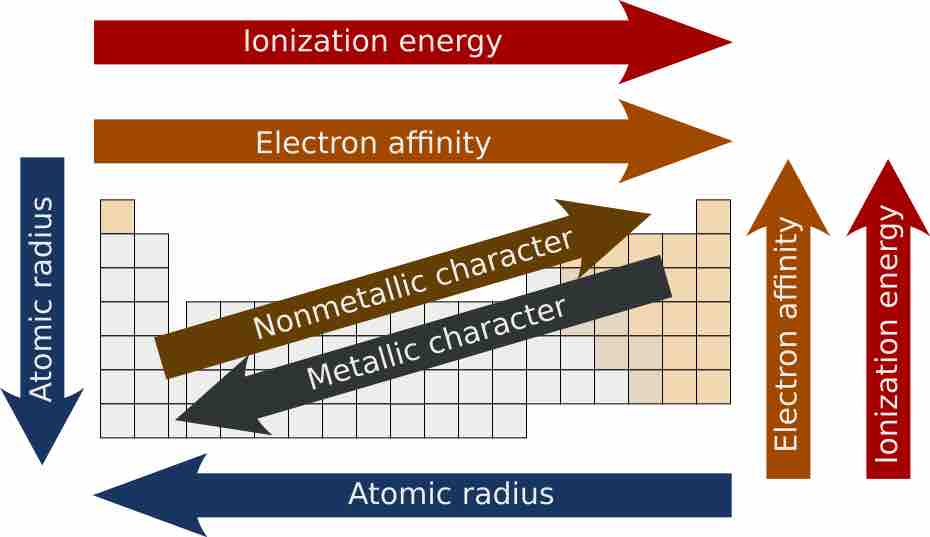
Periodic trends
A graphic showing overall periodic trends in the periodic table.
Meaning of the Atomic Radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius.
Depending on context, the term atomic radius may apply only to isolated atoms, or also to atoms in condensed matter, covalently bound in molecules, or in ionized and excited states. The value of an atomic radius may be obtained through experimental measurements or computed with theoretical models. Under some definitions, the value of a radius may depend on the atom's state and context. For our purposes, we are generally looking at atoms in their elemental state.
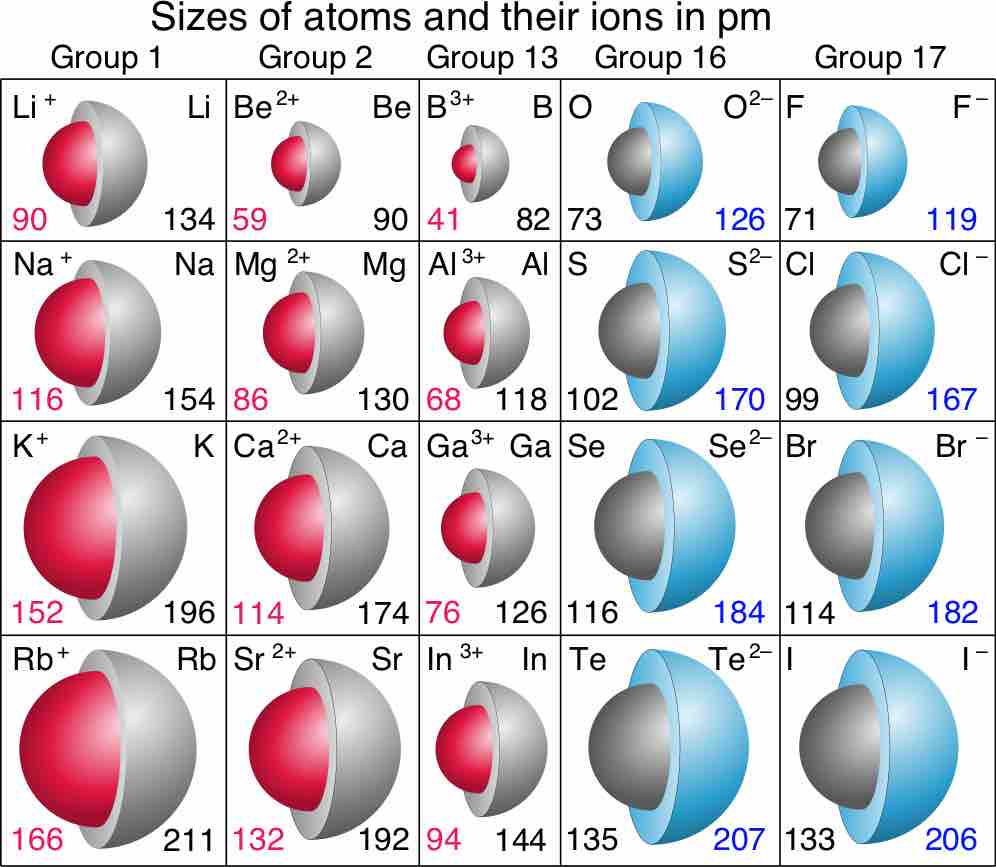
Sizes of atoms and their ions in picometers (pm)
Red numbers are ionic radii of cations, black numbers are for neutral species, and blue numbers are for anions.
Atomic radii vary in a predictable and explicable manner across the periodic table. Radii generally decrease from left to right along each period (row) of the table, from the alkali metals to the noble gases; radii increase down each group (column). The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom. Radii measurements provided important evidence for the development and confirmation of quantum theory.
Explanation of the General Trends
The way atomic radius varies with increasing atomic number can be explained by the arrangement of electrons in shells of fixed capacity. Shells closer to the nucleus—those with a smaller radius—are generally filled first, since the negatively charged electrons are attracted by the positively charged protons in the nucleus. As the atomic number increases along a row of the periodic table, additional electrons are added to the same, outermost shell. The radius of this shell gradually contracts as the attraction between the additional electrons and the nucleus increases. In a noble gas, the outermost shell is completely filled. Therefore, the additional electron of next alkali metal (one row down on the periodic table) will go into a new outer shell, accounting for the sudden increase in the atomic radius.
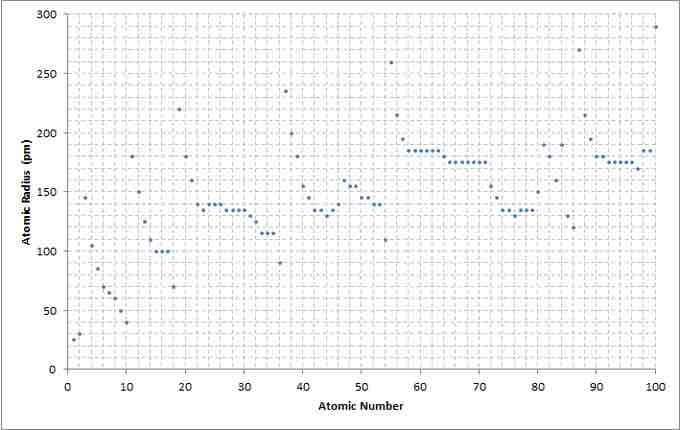
Atomic number to radius graph
A chart showing the atomic radius relative to the atomic number of the elements.
The increasing nuclear charge is partly counterbalanced by the increasing number of electrons, a phenomenon that is known as shielding; this explains why the size of atoms usually increases down each column. Underlying causes of the periodic trends in atomic radius also have an impact on other chemical and physical properties of the elements.