Atoms Are Built Up by Adding Electrons
Although the nucleus of an atom is very dense, the electrons around it can take on a variety of positions which can be summarized as an electron configuration. An element's electron configuration can be represented using energy level diagrams, or Aufbau diagrams. The Aufbau principle (from the German Aufbau meaning "building up, construction") describes a model-building method in which an atom is "built up" by progressively adding electrons. As electrons are added, they assume the most stable shells with respect to the nucleus and the electrons already present.
Filling in an Aufbau Diagram
The order in which orbitals are filled is given by the Madelung rule. The rule is based on the total number of nodes in the atomic orbital, n + ℓ, which is related to the energy. In this context, n represents the principal quantum number and ℓ represents the azimuthal quantum number. The values ℓ = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. According to the principle, electrons fill orbitals starting at the lowest available energy states before filling higher states (e.g., 1s before 2s).

The Madelung energy ordering rule
Order in which orbitals are arranged by increasing energy according to the Madelung Rule. Each diagonal read arrow corresponds to a different value of n + l.
An Aufbau diagram uses arrows to represent electrons. When there are two electrons in an orbital, the electrons are called an electron pair. Electron pairs are shown with arrows pointing in opposite directions. According to the Pauli Exclusion Principle, two electrons in an orbital will not spin the same way. That is, an Aufbau diagram uses arrows pointing in opposite directions. An arrow pointing up denotes an electron spinning one way and an arrow pointing downwards denotes an electron spinning the other way. If the orbital only has one electron, this electron is called an unpaired electron.

Aufbau diagram for lithium
The electron configuration of lithium, shown on an Aufbau diagram
The following steps detail how to draw an Aufbau diagram:
- Determine the number of electrons that the atom has.
- Fill the s orbital in the first energy level (the 1s orbital) with the first two electrons.
- Fill the s orbital in the second energy level (the 2s orbital) with the second two electrons.
- Put one electron in each of the three p orbitals in the second energy level (the 2p orbitals) and then if there are still electrons remaining, go back and place a second electron in each of the 2p orbitals to complete the electron pairs.
- Continue in this way through each of the successive energy levels until all the electrons have been drawn.
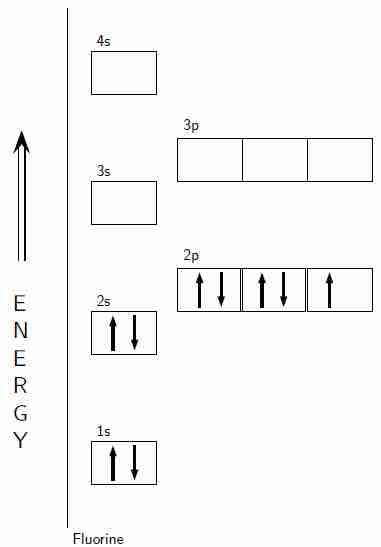
Aufbau diagram for fluorine
An Aufbau diagram showing the electron configuration of fluorine.
Electron Configuration Standard Notation
A special type of notation is used to write an atom's electron configuration. The notation describes the energy levels, orbitals, and the number of electrons in each. For example, the electron configuration of lithium is 1s22s1. The number and letter describe the energy level and orbital, and the number above the orbital shows how many electrons are in that orbital. Using standard notation, the electron configuration of fluorine is 1s22s22p5.
Limitations to Aufbau
The Aufbau principle is based on the idea that the order of orbital energies is fixed—both for a given element and between different elements. This assumption is approximately true—enough for the principle to be useful—but not physically reasonable. It models atomic orbitals as "boxes" of fixed energy into which at most two electrons can be placed. However, the energy of an electron in an atomic orbital depends on the energies of all the other electrons of the atom.
In a hydrogen-like atom, which only has one electron, the s-orbital and the p-orbitals of the same shell in the Aufbau diagram have exactly the same energy. However, in a real hydrogen atom, the energy levels are slightly split by the magnetic field of the nucleus. Because each atom has a different number of protons in its nucleus, the magnetic field differs, which alters the pull on each electron. In general, the Aufbau principle works very well for the ground states of the atoms for the first 18 elements, then decreasingly well for the following 100 elements.