Section 2
Electron Configuration
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
5 concepts
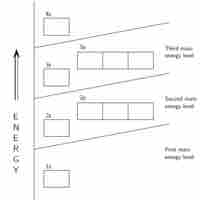
General Rules for Assigning Electrons to Atomic Orbitals
An atom's electrons exist in discrete atomic orbitals, and the atom's electron configuration can be determined using a set of guidelines.

The Building-Up (Aufbau) Principle
The Aufbau principle determines an atom's electron configuration by adding electrons to atomic orbitals following a defined set of rules.
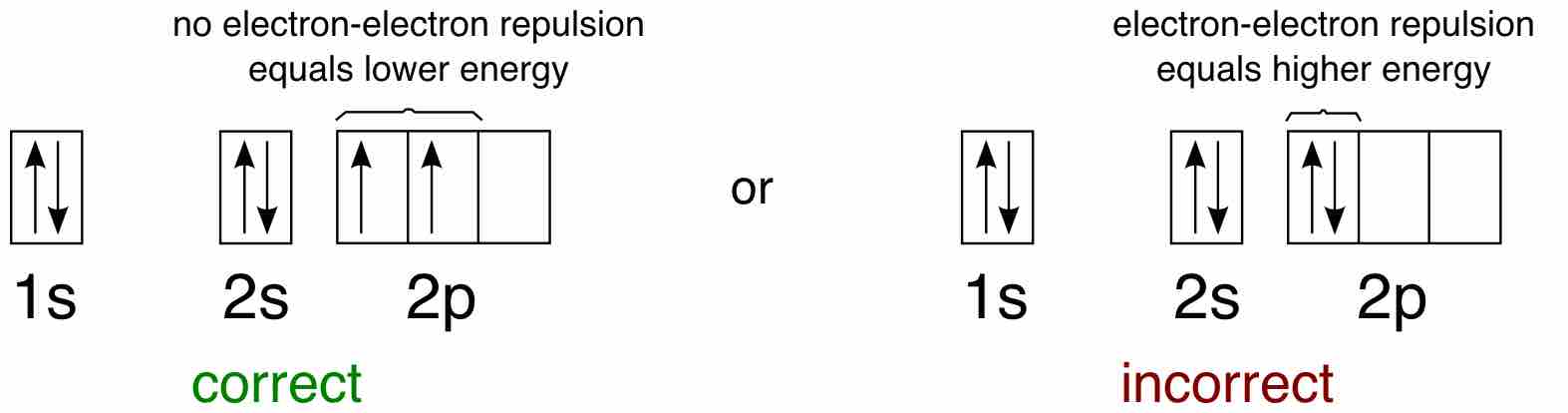
Hund's Rule
Hund's Rule defines the behavior of unpaired valence shell electrons, providing insight into an atom's reactivity and stability.

The Shielding Effect and Effective Nuclear Charge
The shielding effect, approximated by the effective nuclear charge, is due to inner electrons shielding valence electrons from the nucleus.

Diamagnetism and Paramagnetism
Diamagnetic atoms have only paired electrons, whereas paramagnetic atoms, which can be made magnetic, have at least one unpaired electron.