Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases. For some organic sulfur compounds, smell depends on their concentration. The sulfur-containing monoterpenoid grapefruit mercaptan has the characteristic scent of grapefruit in small concentrations, but has a unpleasant thiol odor at larger concentrations.
Reactions with Hydrogen
Treatment of sulfur with hydrogen produces hydrogen sulfide. When dissolved in water, hydrogen sulfide is mildly acidic:
Hydrogen sulfide gas and the hydrosulfide anion are extremely toxic to mammals, because they inhibit the oxygen-carrying capacity of hemoglobin and certain cytochromes in a manner similar to cyanide and azide.
Reduction of Sulfur
Reduction of elemental sulfur produces polysulfides, which consist of chains of sulfur atoms terminated with S– centers:
This reaction highlights arguably the single most distinctive property of sulfur: its ability to catenate (bind to itself by formation of chains). Protonation of these polysulfide anions gives the polysulfanes, H2Sx where x = 2, 3, and 4.
Ultimately, reduction of sulfur gives sulfide salts:
The interconversion of these species is used in sodium-sulfur batteries.
The radical anion S3– gives the blue color of the mineral lapis lazuli . With very strong oxidants, S8 can be oxidized, for example, to give bicyclic S82+.

Lapis lazuli
Lapis lazuli owes its blue color to a sulfur radical.
Sulfur Oxides
The principal sulfur oxides are obtained by burning sulfur:
Other oxides are known—sulfur monoxide and disulfur mono- and dioxides—but they are unstable. The sulfur oxides form numerous oxyanions with the formula SOn2–. Sulfur dioxide and sulfites (SO32−) are related to the unstable sulfurous acid (H2SO3). Sulfur trioxide and sulfates (SO42−) are related to sulfuric acid. Sulfuric acid and SO3 combine to give oleum, a solution of pyrosulfuric acid (H2S2O7) in sulfuric acid.
Peroxides convert sulfur into unstable compounds such as S8O, a sulfoxide. Peroxymonosulfuric acid (H2SO5) and peroxydisulfuric acids (H2S2O8) are made from the action of SO3 on concentrated H2O2, and H2SO4 on concentrated H2O2, respectively. Thiosulfate salts(S2O32−), sometimes referred as "hyposulfites," are used in photographic fixing (HYPO) and as reducing agents. These salts feature sulfur in two oxidation states. Sodium dithionite, (S2O42−) contains the more highly reducing dithionite anion; sodium dithionate (Na2S2O6) is the first member of the polythionic acids (H2SnO6), where n can range from 3 to many.
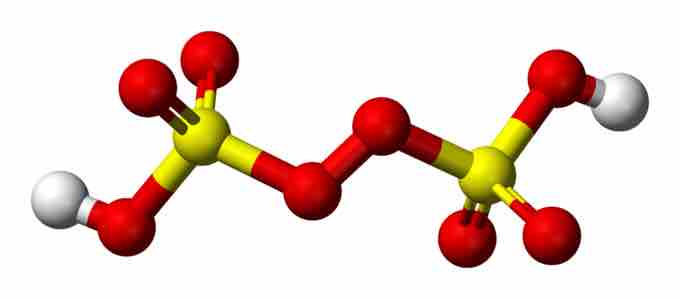
Peroxydisulfuric acid
Peroxydisulfuric acid (H2S2O8) is made from the action of H2SO4 on concentrated H2O2.
Sulfur Halides
There are two main sulfur fluorides. Sulfur hexafluoride is a dense gas used as a nonreactive and nontoxic propellant. Sulfur tetrafluoride is a rarely used organic reagent that is highly toxic. Their chlorinated analogs are sulfur dichloride and sulfur monochloride. Sulfuryl chloride and chlorosulfuric acid are derivatives of sulfuric acid; thionyl chloride (SOCl2) is a common reagent in organic synthesis.
Sulfur-Nitrogen Compounds
An important S–N compound is the cage tetrasulfur tetranitride (S4N4). Heating this compound gives polymeric sulfur nitride ((SN)x), which has metallic properties even though it does not contain any metal atoms. Thiocyanates contain the SCN− group. Oxidation of thiocyanate gives thiocyanogen, (SCN)2 with the connectivity NCS–SCN. Phosphorus sulfides are numerous.
Sulfur with Metals
The principal ores of copper, zinc, nickel, cobalt, molybdenum, and other metals are sulfides. These materials tend to be dark-colored semiconductors that are not readily attacked by water or even many acids. They are formed by the reaction of hydrogen sulfide with metal salts. The mineral galena (PbS) was the first demonstrated semiconductor. It was used as a signal rectifier in the cat's whiskers of early crystal radios. Upgrading these ores, usually by roasting, is costly and environmentally hazardous. Sulfur corrodes many metals via the process called tarnishing.
Organic Compounds with Sulfur
The following are some of the main classes of sulfur-containing organic compounds:
- Thiols or mercaptans (as they are mercury capturers as chelators) are the sulfur analogs of alcohols; treatment of thiols with base gives thiolate ions.
- Thioethers are the sulfur analogs of ethers. Sulfonium ions have three groups attached to a cationic sulfur center. Dimethylsulfoniopropionate (DMSP) is one such compound, important in the marine organic sulfur cycle.
- Sulfoxides and sulfones are thioethers with one and two oxygen atoms attached to the sulfur atom, respectively. The simplest sulfoxide, dimethyl sulfoxide, is a common solvent; a common sulfone is sulfolane. Sulfonic acids are used in many detergents.
- Compounds with carbon–sulfur bonds are uncommon except for carbon disulfide, a volatile colorless liquid that is structurally similar to carbon dioxide. Unlike carbon monoxide, carbon monosulfide is only stable as a dilute gas, as in the interstellar medium. Organosulfur compounds are responsible for the some of the unpleasant odors of decaying organic matter.
Vulcanization
Sulfur-sulfur bonds are a structural component to stiffen rubber, similar to the biological role of disulfide bridges in rigidifying proteins. In the most common type of industrial "curing" or hardening and strengthening of natural rubber, elemental sulfur is heated with the rubber until chemical reactions form disulfide bridges between isoprene units of the polymer. Because of the heat and sulfur, the process was named vulcanization, after the Roman god of the forge and volcanism.