Silicate Tetrahedrons
The basic building block of all silicate minerals is the [SiO4]4− tetrahedron. There are four covalent Si−O bonds. Each oxygen atom forms one vertex of the tetrahedron. The silicon to oxygen atom ratio is 1:4.
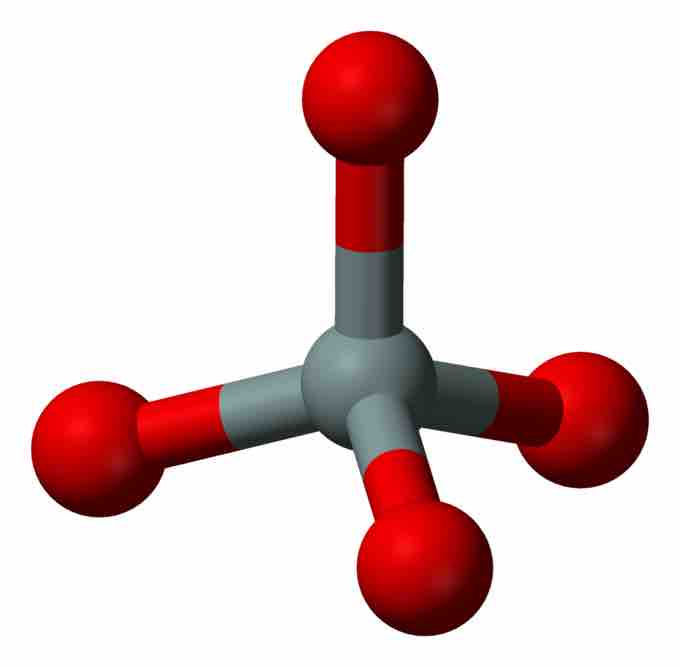
Silicate Tetrahedron
Ball-and-stick model of the silicate tetrahedron; red represents oxygen atoms and gray represents the silicon atom in the center.
Silicate minerals containing isolated [SiO4]4− tetrahedrons are called nesosilicates or orthosilicates.
Corner-Sharing Tetrahedrons
If two [SiO4]4− tetrahedrons share an oxygen atom at one common vertex, an [Si2O7]6− ion is formed. The silicon to oxygen ratio is 2:7. Silicate minerals containing isolated [Si2O7]6− double tetrahedrons are called sorosilicates.
Silicate Chains
Silicate minerals containing chains are termed inosilicates. They consist of single chains (SiO32−)n, in which the silicon to oxygen atom ratio is 1:3, and double chains (Si4O116−)n, in which the silicon to oxygen atom ratio is 4:11.
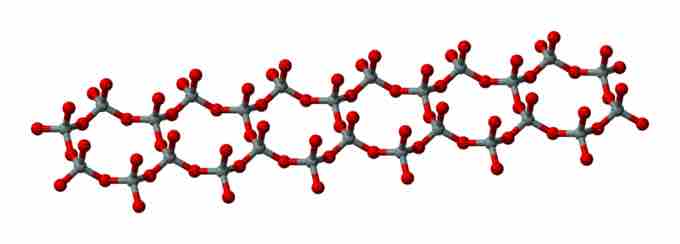
Double Chain
Ball-and-stick model of silicate double chains. Red balls correspond to oxygen, and gray to silicon atoms.
Asbestos
Asbestos (from Greek ἅ, unquenchable) is a group of fibrous silicate minerals containing double chains. Prolonged exposure to dust containing fibres from certain types of asbestos is now known to cause scarring of the lungs, lung cancer, and a particularly aggressive cancer called mesothelioma. Mesothelioma is almost always fatal, with a median survival time of 11 months. Due to the exceptional danger posed by some absestos, certain counties now require all work involving asbestos to be done by specialist companies. The vast majority of asbestos is the so-called white form, which is not known to pose any real danger.
Silicate Sheets
SiO4 tetrahedrons can be arranged to form sheets. The formula of such a sheet can be written (Si2O52−)n. Silicate minerals containing sheets are termed phyllosilicates.
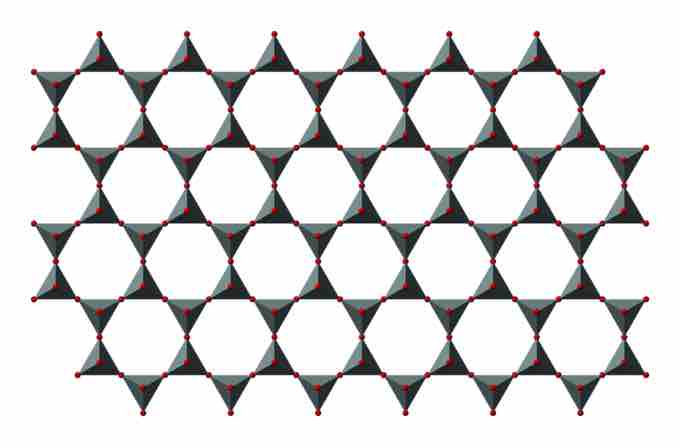
Silicate Sheet
The formula of silicate sheet is (Si2O52−)n.
Three-dimensional Frameworks
Perhaps the most structurally complicated silicates are those based on networks of Si and O that extend in all three dimensions. Examples of such minerals include quartz, zeolites, and feldspars. Silicate minerals containing three-dimensional frameworks are termed tectosilicates.
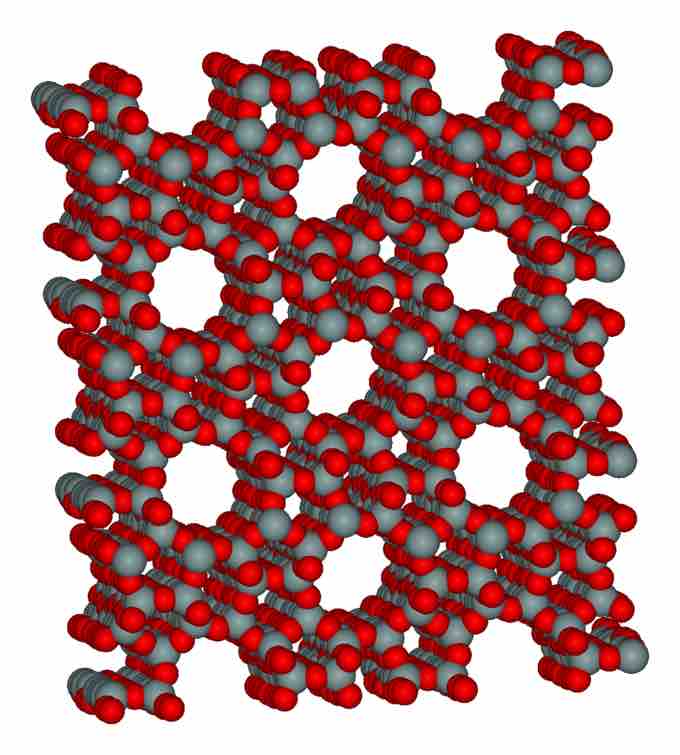
Three-dimensional structure of zeolite
In the mineral zeolite, silica and oxygen atoms are bonded layers of sheets.