Oxygen is an important part of the atmosphere and is necessary to sustain terrestrial life. Because it comprises most of the mass in water, it also comprises most of the mass of living organisms. All major classes of structural molecules in living organisms, such as proteins, carbohydrates, and fats, contain oxygen, as do the major inorganic compounds that comprise animal shells, teeth, and bone. Elemental oxygen (O2) is produced by cyanobacteria, algae, and plants through the process of photosynthesis, and is used in cellular respiration by most living organisms on earth. Oxygen is toxic to obligate anaerobic organisms (organisms which need a lack of oxygen for survival), which were the dominant form of early life on Earth, until O2 began to accumulate in the atmosphere.
Chemical Properties of Oxygen
At standard temperature and pressure (STP), two atoms of the element bind to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula O2. Oxygen is a member of the chalcogen group on the periodic table and is a highly reactive nonmetallic element. As such, it readily forms compounds (notably, oxides) with almost all other elements. Oxygen is a strong oxidizing agent and has the second-highest electronegativity of all reactive elements, second only to fluorine. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium, and the most abundant element by mass in the Earth's crust, making up almost half of the crust's mass. Free oxygen is too chemically reactive to appear on Earth without the photosynthetic action of living organisms, which use the energy of sunlight to produce elemental oxygen from water. Elemental O2 only began to accumulate in the atmosphere after the evolutionary appearance of photosynthetic organisms, roughly 2.5 billion years ago. Diatomic oxygen gas currently constitutes 20.8 percent of the volume of air.
Diatomic Oxygen
The two oxygen atoms in diatomic oxygen are chemically bonded to each other with a spin triplet electron configuration. This bond has a bond order of two and is often simplified in descriptions as a double bond, or as a combination of one two-electron bond and two three-electron bonds. Triplet oxygen (not to be confused with ozone, O3) is the ground state of the O2 molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals. These orbitals are classified as antibonding (weakening the bond order from three to two), so the diatomic oxygen bond is weaker than the diatomic nitrogen triple bond, in which all bonding molecular orbitals are filled, but some antibonding orbitals are not.
In normal triplet form, O2 molecules are paramagnetic. This means they behave as magnets in the presence of an external magnetic field, because of the spin magnetic moments of the unpaired electrons in the molecule. Liquid oxygen is attracted to a magnet to a sufficient extent that, in laboratory demonstrations, a bridge of liquid oxygen may be supported against its own weight between the poles of a powerful magnet. Singlet oxygen is a name given to several higher-energy species of molecular O2 in which all the electron spins are paired. It is much more reactive toward common organic molecules than is the triplet form of molecular oxygen.
Physical Properties of Oxygen
Oxygen is more soluble in water than nitrogen is; water contains approximately one molecule of O2 for every two molecules of N2, compared to an atmospheric ratio of approximately one to four. The solubility of oxygen in water is temperature-dependent, and about twice as much (14.6 mg/L) dissolves at 0 °C than at 20 °C (7.6 mg/L). At 25 °C and 1 standard atmosphere (101.3 kPa) of air, freshwater contains about 6.04 milliliters (mL) of oxygen per liter, whereas seawater contains about 4.95 mL per liter. At 5 °C the solubility increases to 9.0 mL (50 percent more than at 25 °C) per liter for water and 7.2 mL (45 percent more) per liter for sea water.
Oxygen condenses at 90.20 K (−182.95 °C, −297.31 °F), and freezes at 54.36 K (−218.79 °C, −361.82 °F). Both liquid and solid O2 are clear substances with a light sky-blue color caused by absorption in the red (in contrast with the blue color of the sky, which is due to Rayleigh scattering of blue light) .
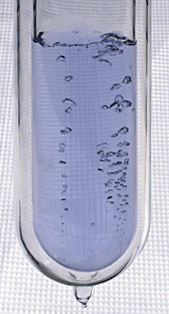
Liquid Oxygen
Oxygen bubbles rise through pale-blue liquid oxygen.
High-purity liquid O2 is usually obtained by the fractional distillation of liquefied air. Liquid oxygen may also be produced by condensation out of air, using liquid nitrogen as a coolant. It is a highly reactive substance and must be segregated from combustible materials.