The element nitrogen was discovered as a separable component of air by Scottish physician Daniel Rutherford in 1772. Nitrogen compounds were well known during the Middle Ages. Alchemists knew nitric acid as aqua fortis (strong water). The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), celebrated for its ability to dissolve gold (the king of metals). The earliest military, industrial, and agricultural applications of nitrogen compounds used saltpetre (sodium nitrate or potassium nitrate), most notably in gunpowder and later as fertilizer.
Nitrogen is a chemical element with symbol N and atomic number 7. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.09 percent of Earth's atmosphere by volume. Nitrogen is a common element in the universe, estimated at about seventh in total abundance in our galaxy and the solar system. Its occurrence there is thought to be entirely due to synthesis by fusion of carbon and hydrogen in supernovas. Due to the volatility of elemental nitrogen and its compounds with hydrogen and oxygen, nitrogen is far less common on the rocky planets of the inner solar system and is a relatively rare element on Earth. However, as on Earth, nitrogen and its compounds occur commonly as gases in the atmospheres of planets and moons.
Nitrogen in Living Systems
Nitrogen occurs in all living organisms, primarily in amino acids which make up proteins, and nucleic acids (DNA and RNA). The human body is about three percent nitrogen by weight, the fourth-most abundant element after oxygen, carbon, and hydrogen. Nitrogen resides in the chemical structure of almost all neurotransmitters and is a defining component of alkaloids, biological molecules produced as secondary metabolites by many organisms.
The nitrogen cycle describes the movement of the element from the air into the biosphere and organic compounds and back into the atmosphere. Synthetically produced nitrates are key ingredients of industrial fertilizers and key pollutants causing the eutrophication of water systems.
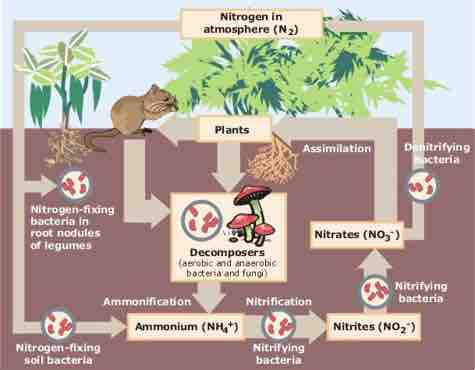
The Nitrogen Cycle
The figure summarizes the major processes through which nitrogen is converted between its various forms on the surface of the earth.
Industrial Production of Nitrogen
Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air or by mechanical means using gaseous air (i.e., pressurized reverse osmosis membrane or pressure swing adsorption). Commercial nitrogen is often a byproduct of air-processing for industrial concentration of oxygen for steelmaking and other purposes. When supplied compressed in cylinders, it is often called OFN (oxygen-free nitrogen).
In a chemical laboratory it is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite, or through the decomposition of sodium azide:
Chemical Properties of Nitrogen
Nitrogen is a nonmetal with an electronegativity of 3.04. It has five electrons in its outer shell and is, therefore, trivalent in most compounds. The triple bond in molecular nitrogen (N2) is one of the strongest known. The resulting difficulty of converting N2 into other compounds, and the ease (and associated high energy release) of converting nitrogen compounds into elemental N2, have dominated the role of nitrogen in both nature and human economic activities.
Nitrogen Emission Spectrum
Molecular nitrogen (14N2) is largely transparent to infrared and visible radiation because it is a homonuclear molecule and, therefore, has no dipole moment to couple the electromagnetic radiation at these wavelengths. Significant absorption occurs at extreme ultraviolet wavelengths, beginning at a wavelength of around 100 nanometers. This is associated with electronic transitions in the molecule to states in which charge is not distributed evenly between nitrogen atoms. Nitrogen absorption leads to significant absorption of ultraviolet radiation in the Earth's upper atmosphere and the atmospheres of other planetary bodies.

Spectrum of Nitrogen
Sending an electric current through nitrogen excites the electrons to higher energy levels. When they fall to lower energy levels, certain frequencies of light (based on the difference in energy of the energy levels) are observed, as shown.
Applications of Nitrogen Gas
Nitrogen gas has a variety of applications, including:
- As an inert replacement for air where oxidation is undesirable
- As a modified atmosphere, pure or mixed with carbon dioxide, to preserve the freshness of packaged or bulk foods
- In ordinary incandescent light bulbs as an inexpensive alternative to argon
- In production of electronic parts such as transistors, diodes, and integrated circuits
- Filling automotive and aircraft tires due to its inertness and lack of moisture or oxidative qualities, as compared to air
- As a propellant for draft wine, and as an alternative to or in combination with carbon dioxide in carbonated beverages
Nitrogen is also used in preparing samples for chemical analysis to concentrate and reduce the volume of liquid samples. Directing a pressurized stream of nitrogen gas perpendicular to the surface of the liquid allows the solvent to evaporate while leaving the solute(s) and unevaporated solvent behind. Nitrogen tanks are also replacing carbon dioxide as the main power source for paintball guns. But, nitrogen must be kept at higher pressure than CO2, making N2 tanks heavier and more expensive.