Metallic Bonding
Metallic bonding may be described as the sharing of free electrons among a lattice of positively charged metal ions. The structure of metallic bonds is very different from that of covalent and ionic bonds. While ionic bonds join metals to nonmetals, and covalent bonds join nonmetals to nonmetals, metallic bonds are responsible for the bonding between metal atoms.
In metallic bonds, the valence electrons from the s and p orbitals of the interacting metal atoms delocalize. That is to say, instead of orbiting their respective metal atoms, they form a "sea" of electrons that surrounds the positively charged atomic nuclei of the interacting metal ions. The electrons then move freely throughout the space between the atomic nuclei.
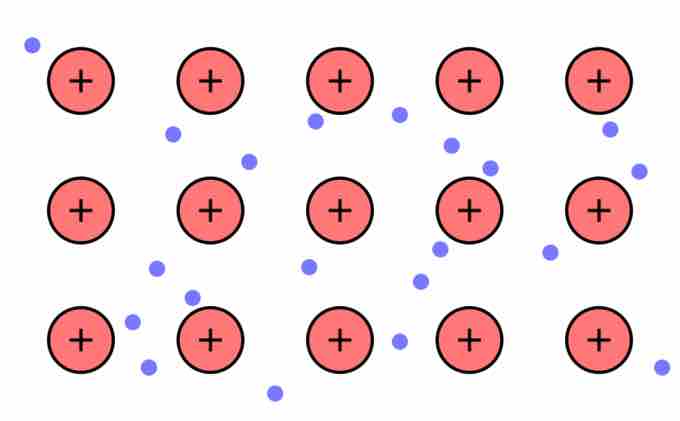
Metallic Bonding: The Electron Sea Model
Positive atomic nuclei surrounded by a sea of delocalized electrons (the blue dots).
The characteristics of metallic bonds explain a number of the unique properties of metals:
- Metals are good conductors of electricity because the electrons in the electron sea are free to flow and carry electric current.
- Metals are ductile and malleable because local bonds can be easily broken and reformed.
- Metals are shiny. Light cannot penetrate their surface; the photons simply reflect off the metal surface. However, there is an upper limit to the frequency of light at which the photons are reflected.
Metallic bonds can occur between different elements, forming an alloy. Aluminum foil and copper wire are examples of metallic bonding in action .

Aluminum foil
A sheet of aluminum foil is made up of metallic bonds.
Metallic bonds are mediated by strong attractive forces. This property contributes to the low volatility, high melting and boiling points, and high density of most metals. The group-XII metals zinc, cadmium, and mercury are exceptions to this rule.