Pure water cannot undergo significant electrolysis without adding an electrolyte. If the object is to produce hydrogen and oxygen, the added electrolyte must be energetically more difficult to oxidize or reduce than water itself. For example, electrolysis of a solution of sulfuric acid or of a salt, such as NaNO3, results in the decomposition of water at both electrodes:
Cathode:
E = 0.00 V
Anode:
E° = -1.23 V
Multiplying the cathode reaction by 2, in order to match the number of electrons transferred, results in this net equation, after OH- and H+ ions combine to form water:
Net:
E = -1.23 v
Hydrogen will appear at the cathode, the negatively charged electrode, where electrons enter the water, and oxygen will appear at the anode, the positively charged electrode. The number of moles of hydrogen generated is twice the number of moles of oxygen, and both are proportional to the total electrical charge conducted by the solution. The number of electrons pushed through the water is twice the number of generated hydrogen molecules, and four times the number of generated oxygen molecules.
Johann Ritter, who went on to invent the first electrochemical cell, was one of the first people to discover the decomposition of water by electricity .
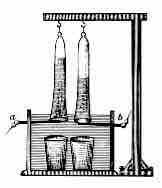
Electrolysis of water
Device invented by Johann Wilhelm Ritter to develop the electrolysis of water.