In chemistry and manufacturing, electrolysis is a method of using a direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the process of separating elements from naturally occurring sources such as ore.
Electrolysis is the passage of a direct electric current through an ionic substance that is either molten or dissolved in a suitable solvent, resulting in chemical reactions at the electrodes and separation of the materials.
Electrolysis can sometimes be thought of as running a non-spontaneous galvanic cell. Depending on how freely elements give up electrons (oxidation) and how energetically favorable it is for elements to receive electrons (reduction), the reaction may not be spontaneous. By externally supplying the energy to overcome the energy barrier to spontaneous reaction, the desired reaction is "allowed" to run under special circumstances.
The main components required to perform electrolysis are:
- An electrolyte: a substance containing free ions that carry electric current. If the ions are not mobile, as in a solid salt, then electrolysis cannot occur.
- A direct current (DC) supply: provides the energy necessary to create or discharge the ions in the electrolyte. Electric current is carried by electrons in the external circuit.
- Two electrodes: an electrical conductor that provides the physical interface between the electrical circuit providing the energy and the electrolyte.
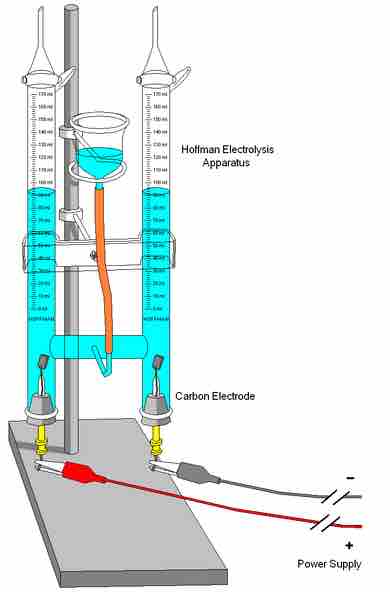
A typical electrolysis cell
A cell used in elementary chemical experiments to produce gas as a reaction product and to measure its volume.
Electrodes of metal, graphite, and semiconductor material are widely used. Choosing a suitable electrode depends on the chemical reactivity between the electrode and electrolyte, and the cost of manufacture.
Other systems that utilize the electrolytic process are used to produce metallic sodium and potassium, chlorine gas, sodium hydroxide, and potassium and sodium chlorate.