Defining a Dry Cell
In electricity, a battery is a device consisting of one or more electrochemical cells that convert stored chemical energy into electrical energy. The dry cell is one of many general types of electrochemical cells.
A dry cell has the electrolyte immobilized as a paste, with only enough moisture in it to allow current to flow. Unlike a wet cell, a dry cell can operate in any orientation without spilling, as it contains no free liquid. This versatility makes it suitable for portable equipment. By comparison, the first wet-cell batteries were typically fragile glass containers with lead rods hanging from an open top. They, therefore, needed careful handling to avoid spillage. The development of the dry-cell battery allowed for a major advance in battery safety and portability.
A common dry-cell battery is the zinc-carbon battery, which uses a cell that is sometimes called the Leclanché cell. The cell is made up of an outer zinc container, which acts as the anode. The cathode is a central carbon rod, surrounded by a mixture of carbon and manganese(IV) dioxide (MnO2). The electrolyte is a paste of ammonium chloride (NH4Cl). A fibrous fabric separates the two electrodes, and a brass pin in the center of the cell conducts electricity to the outside circuit.
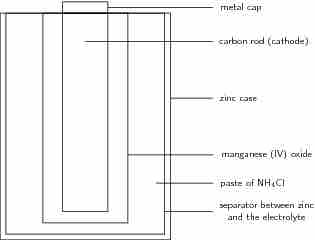
A zinc-carbon dry-cell battery
An illustration of a zinc-carbon dry cell. In it, a zinc casing acts as the anode, surrounding a carbon rod, which acts as a cathode. Between them, the electrolyte paste works as the battery.
Chemical reactions occur in every part of the battery to allow for energy storage; the reactions can be described using balanced chemical equations that delineate the electron flow. The paste of ammonium chloride reacts according to the following half-reaction:
The manganese(IV) oxide in the cell removes the hydrogen produced by the ammonium chloride, according to the following reaction:
The combined result of these two reactions takes place at the cathode. Adding these two reactions together, we get:
Finally, the anode half-reaction is as follows:
Therefore, the overall equation for the cell is:
The potential for the above reaction is 1.50 V.
Another example of a dry-cell battery is the alkaline battery. Alkaline batteries are almost the same as zinc-carbon batteries, except that the electrolyte used is potassium hydroxide (KOH) rather than ammonium chloride. In some more modern types of so-called "high-power" batteries that have a much lower capacity than standard alkaline batteries, the ammonium chloride is replaced by zinc chloride.