Reactant Concentrations
Raising the concentrations of reactants makes the reaction happen at a faster rate. For a chemical reaction to occur, there must be a certain number of molecules with energies equal to or greater than the activation energy. With an increase in concentration, the number of molecules with the minimum required energy will increase, and therefore the rate of the reaction will increase. For example, if one in a million particles has sufficient activation energy, then out of 100 million particles, only 100 will react. However, if you have 200 million of those particles within the same volume, then 200 of them react. By doubling the concentration, the rate of reaction has doubled as well.
Surface Area
In a reaction between a solid and a liquid, the surface area of the solid will ultimately impact how fast the reaction occurs. This is because the liquid and the solid can bump into each other only at the liquid-solid interface, which is on the surface of the solid. The solid molecules trapped within the body of the solid cannot react. Therefore, increasing the surface area of the solid will expose more solid molecules to the liquid, which allows for a faster reaction.
For example, consider a 6 x 6 x 2 inch brick. The area of the exposed surfaces of the brick is
This shows that the total exposed surface area will increase when a larger body is divided into smaller pieces. Therefore, since a reaction takes place on the surface of a substance, increasing the surface area should increase the quantity of the substance that is available to react, and will thus increase the rate of the reaction as well.
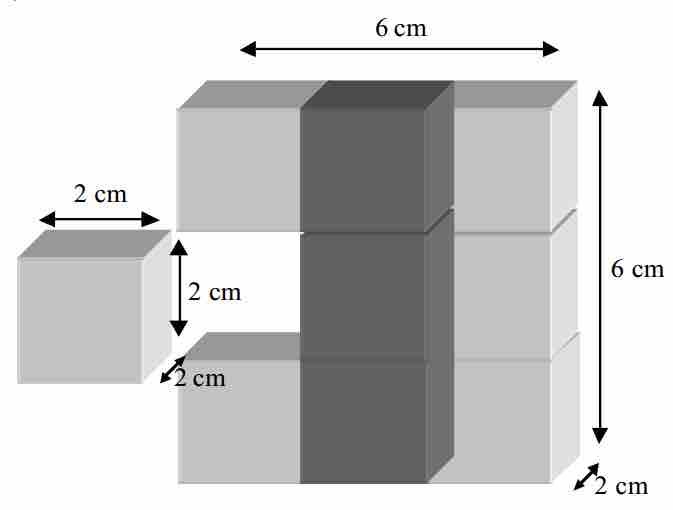
Surface areas of smaller molecules versus larger molecules
This picture shows how dismantling a brick into smaller cubes causes an increase in the total surface area.
Pressure
Increasing the pressure for a reaction involving gases will increase the rate of reaction. As you increase the pressure of a gas, you decrease its volume (PV=nRT; P and V are inversely related), while the number of particles (n) remains unchanged. Therefore, increasing pressure increases the concentration of the gas (n/V), and ensures that the gas molecules collide more frequently. Keep in mind this logic only works for gases, which are highly compressible; changing the pressure for a reaction that involves only solids or liquids has no effect on the reaction rate.
Temperature
It has been observed experimentally that a rise of 10 °C in temperature usually doubles or triples the speed of a reaction between molecules. The minimum energy needed for a reaction to proceed, known as the activation energy, stays the same with increasing temperature. However, the average increase in particle kinetic energy caused by the absorbed heat means that a greater proportion of the reactant molecules now have the minimum energy necessary to collide and react. An increase in temperature causes a rise in the energy levels of the molecules involved in the reaction, so the rate of the reaction increases. Similarly, the rate of reaction will decrease with a decrease in temperature.
Presence or Absence of a Catalyst
Catalysts are substances that increase reaction rate by lowering the activation energy needed for the reaction to occur. A catalyst is not destroyed or changed during a reaction, so it can be used again. For example, at ordinary conditions, H2 and O2 do not combine. However, they do combine in the presence of a small quantity of platinum, which acts as a catalyst, and the reaction then occurs rapidly.
Nature of the Reactants
Substances differ markedly in the rates at which they undergo chemical change. The differences in reactivity between reactions may be attributed to the different structures of the materials involved; for example, whether the substances are in solution or in the solid state matters. Another factor has to do with the relative bond strengths within the molecules of the reactants. For example, a reaction between molecules with atoms that are bonded by strong covalent bonds will take place at a slower rate than would a reaction between molecules with atoms that are bonded by weak covalent bonds. This is due to the fact that it takes more energy to break the bonds of the strongly bonded molecules.