Double and Triple Covalent Bonds
Covalent bonding occurs when electrons are shared between atoms. Double and triple covalent bonds occur when four or six electrons are shared between two atoms, and they are indicated in Lewis structures by drawing two or three lines connecting one atom to another. It is important to note that only atoms with the need to gain or lose at least two valence electrons through sharing can participate in multiple bonds.
Bonding Concepts
Hybridization
Double and triple bonds can be explained by orbital hybridization, or the 'mixing' of atomic orbitals to form new hybrid orbitals. Hybridization describes the bonding situation from a specific atom's point of view. A combination of s and p orbitals results in the formation of hybrid orbitals. The newly formed hybrid orbitals all have the same energy and have a specific geometrical arrangement in space that agrees with the observed bonding geometry in molecules. Hybrid orbitals are denoted as spx, where s and p denote the orbitals used for the mixing process, and the value of the superscript x ranges from 1-3, depending on how many p orbitals are required to explain the observed bonding.
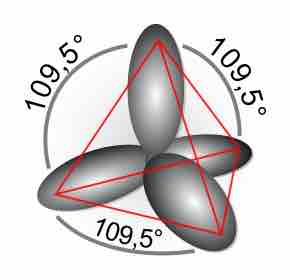
Hybridized orbitals
A schematic of the resulting orientation in space of sp3 hybrid orbitals. Notice that the sum of the superscripts (1 for s, and 3 for p) gives the total number of formed hybrid orbitals. In this case, four orbitals are produced which point along the direction of the vertices of a tetrahedron.
Pi Bonds
Pi, or
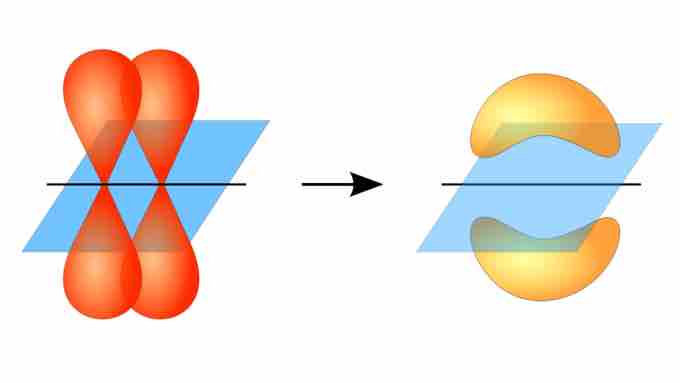
Pi bond formation
Overlap between adjacent unhybridized p orbitals produces a pi bond. The electron density corresponding to the shared electrons is not concentrated along the internuclear axis (i.e., between the two atoms), unlike in sigma bonds.
Multiple bonds between atoms always consist of a sigma bond, with any additional bonds being of the π type.
Examples of Pi Bonds
The simplest example of an organic compound with a double bond is ethylene, or ethene, C2H4. The double bond between the two carbon atoms consists of a sigma bond and a π bond.
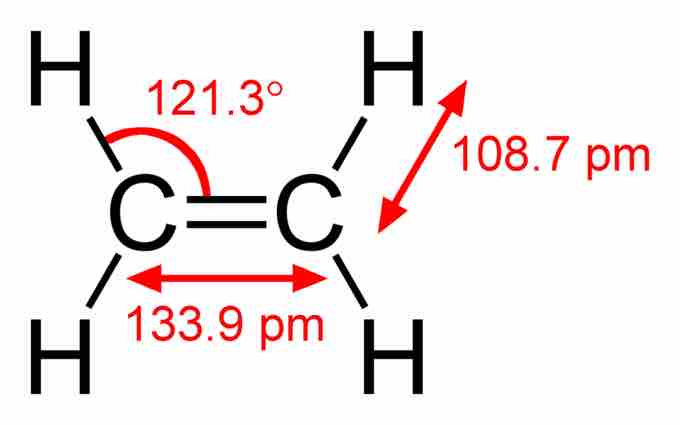
Ethylene bonding
An example of a simple molecule with a double bond between carbon atoms. The bond lengths and angles (indicative of the molecular geometry) are indicated.
From the perspective of the carbon atoms, each has three sp2 hybrid orbitals and one unhybridized p orbital. The three sp2 orbitals lie in a single plane at 120-degree angles. As the carbon atoms approach each other, their orbitals overlap and form a bond. Simultaneously, the p orbitals approach each other and form a bond. To maintain this bond, the p orbitals must stay parallel to each other; therefore, rotation is not possible.
A triple bond involves the sharing of six electrons, with a sigma bond and two
Observable Consequences of Multiple Bonds
Bond Strength
Covalent bonds can be classified in terms of the amount of energy that is required to break them. Based on the experimental observation that more energy is needed to break a bond between two oxygen atoms in O2 than two hydrogen atoms in H2, we infer that the oxygen atoms are more tightly bound together. We say that the bond between the two oxygen atoms is stronger than the bond between two hydrogen atoms.
Experiments have shown that double bonds are stronger than single bonds, and triple bonds are stronger than double bonds. Therefore, it would take more energy to break the triple bond in N2 compared to the double bond in O2. Indeed, it takes 497 kcal/mol to break the O2 molecule, while it takes 945 kJ/mol to do the same to the N2 molecule.
Bond Length
Another consequence of the presence of multiple bonds between atoms is the difference in the distance between the nuclei of the bonded atoms. Double bonds have shorter distances than single bonds, and triple bonds are shorter than double bonds.