The Octet Rule
Noble gases like He, Ne, Ar, Kr, etc., are stable because their valence level is filled with as many electrons as possible. Eight electrons fill the valence level for all noble gases, except helium, which has two electrons in its full valence level. Other elements in the periodic table react to form bonds in which valence electrons are exchanged or shared in order to achieve a valence level which is filled, just like in the noble gases. We refer to this chemical tendency of atoms as 'the octet rule,' and it guides us in predicting how atoms combine to form molecules and compounds.
Covalent Bonds and Lewis Diagrams of Simple Molecules
The simplest example to consider is hydrogen (H), which is the smallest element in the periodic table with one proton and one electron. Hydrogen can become stable if it achieves a full valence level like the noble gas that is closest to it in the periodic table, helium (He). These are exceptions to the octet rule because they only require 2 electrons to have a full valence level.
Two H atoms can come together and share each of their electrons to create a 'covalent bond.' The shared pair of electrons can be thought of as belonging to either atom, and thus each atom now has two electrons in its valence level, like He. The molecule that results is H2, and it is the most abundant molecule in the universe.
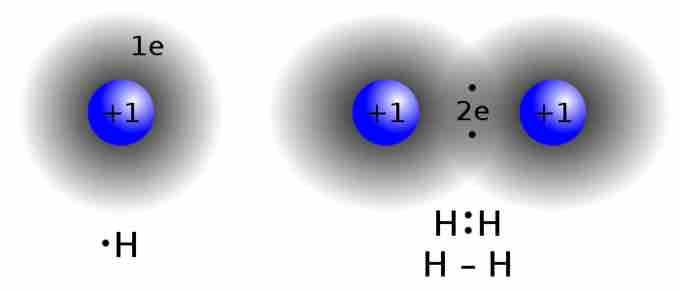
Lewis structure of diatomic hydrogen
This is the process through which the H2 molecule is formed. Two H atoms, each contributing an electron, share a pair of electrons. This is known as a 'single covalent bond.' Notice how the two electrons can be found in a region of space between the two atomic nuclei.
The Lewis formalism used for the H2 molecule is H:H or H—H. The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
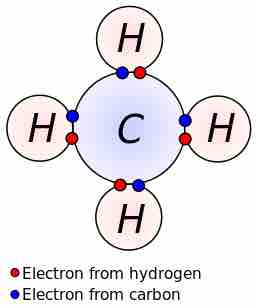
Lewis dot dragram for methane
Methane, with molecular formula CH4, is shown. The electrons are color-coded to indicate which atoms they belonged to before the covalent bonds formed, with red representing hydrogen and blue representing carbon. Four covalent bonds are formed so that C has an octet of valence electrons, and each H has two valence electrons—one from the carbon atom and one from one of the hydrogen atoms.
Now consider the case of fluorine (F), which is found in group VII (or 17) of the periodic table. It therefore has 7 valence electrons and only needs 1 more in order to have an octet. One way that this can happen is if two F atoms make a bond, in which each atom provides one electron that can be shared between the two atoms. The resulting molecule that is formed is F2, and its Lewis structure is F—F.
Achieving an octet of valence electrons
Two fluorine atoms are able to share an electron pair, which becomes a covalent bond. Notice that only the outer (valence level) electrons are involved, and that in each F atom, 6 valence electrons do not participate in bonding. These are 'lone pairs' of electrons.
After a bond has formed, each F atom has 6 electrons in its valence level which are not used to form a bond. These non-bonding valence electrons are called 'lone pairs' of electrons and should always be indicated in Lewis diagrams.
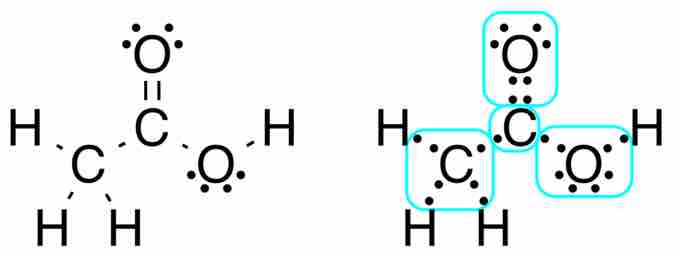
Lewis structure of acetic acid
Acetic acid, CH3COOH, can be written out with dots indicating the shared electrons, or, preferably, with dashes representing covalent bonds. Notice the lone pairs of electrons on the oxygen atoms are still shown. The methyl group carbon atom has six valence electrons from its bonds to the hydrogen atoms because carbon is more electronegative than hydrogen. Also, one electron is gained from its bond with the other carbon atom because the electron pair in the C−C bond is split equally.
Procedure for Drawing Simple Lewis Structures
We have looked at how to determine Lewis structures for simple molecules. The procedure is as follows:
- Write a structural diagram of the molecule to clearly show which atom is connected to which (although many possibilities exist, we usually pick the element with the most number of possible bonds to be the central atom).
- Draw Lewis symbols of the individual atoms in the molecule.
- Bring the atoms together in a way that places eight electrons around each atom (or two electrons for H, hydrogen) wherever possible.
- Each pair of shared electrons is a covalent bond which can be represented by a dash.

Alternate view of lewis dot structure of water
This arrangement of shared electrons between O and H results in the oxygen atom having an octet of electrons, and each H atom having two valence electrons.
Multiple bonds can also form between elements when two or three pairs of electrons are shared to produce double or triple bonds, respectively. The Lewis structure for carbon dioxide, CO2, is a good example of this.

Lewis structure of carbon dioxide
This figure explains the bonding in a CO2 molecule. Each O atom starts out with six (red) electrons and C with four (black) electrons, and each bond behind an O atom and the C atom consists of two electrons from the O and two of the four electrons from the C.
In order to achieve an octet for all three atoms in CO2, two pairs of electrons must be shared between the carbon and each oxygen. Since four electrons are involved in each bond, a double covalent bond is formed. You can see that this is how the octet rule is satisfied for all atoms in this case. When a double bond is formed, you still need to show all electrons, so double dashes between the atoms show that four electrons are shared.
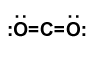
Final Lewis structure for carbon dioxide
Covalent bonds are indicated as dashes and lone pairs of electrons are shown as pairs of dots. in carbon dioxide, each oxygen atom has two lone pairs of electrons remaining; the covalent bonds between the oxygen and carbon atoms each use two electrons from the oxygen atom and two from the carbon.