Concept
Version 18
Created by Boundless
Introduction to Lewis Structures for Covalent Molecules
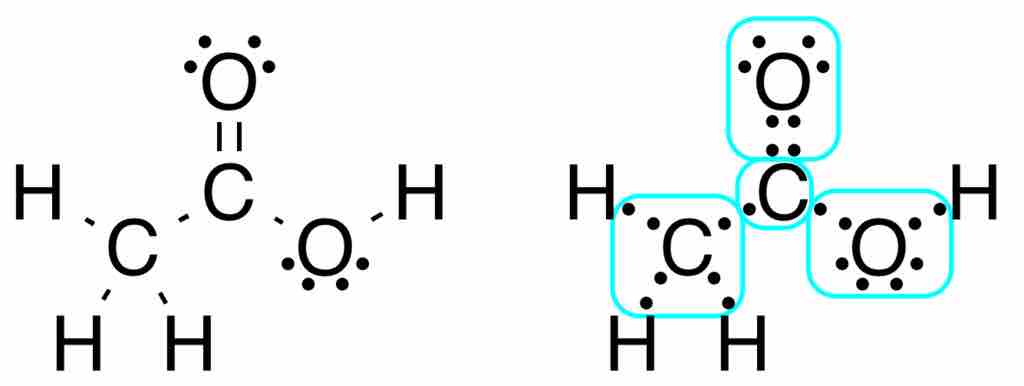
Lewis structure of acetic acid
Acetic acid, CH3COOH, can be written out with dots indicating the shared electrons, or, preferably, with dashes representing covalent bonds. Notice the lone pairs of electrons on the oxygen atoms are still shown. The methyl group carbon atom has six valence electrons from its bonds to the hydrogen atoms because carbon is more electronegative than hydrogen. Also, one electron is gained from its bond with the other carbon atom because the electron pair in the C−C bond is split equally.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Acetic_acid_oxidation_state_analysis.png."
https://commons.wikimedia.org/wiki/File:Acetic_acid_oxidation_state_analysis.png
Wikimedia
Public domain.