Energy Transfer During Breaking or Formation of Bonds
Change in Enthalpy
Enthalpy is a measure of the total heat energy content in a thermodynamic system, and it is practically used to describe energy transfer during chemical or physical processes in which the pressure remains constant.
The total enthalpy, H, of a system cannot be measured directly. Thus, the change in enthalpy,
Generally, a positive change in enthalpy is required to break a bond, while a negative change in enthalpy is accompanied by the formation of a bond. In other words, breaking a bond is an endothermic process, while the formation of bonds is exothermic.
Bond Enthalpy or Dissociation Energy
Bond enthalpy, also known as bond dissociation energy, is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K (absolute zero).
.jpg)
Homolysis of a chemical bond
A two-electron covalent bond is equally split when bond breaking, with each resulting fragment having one electron from the original shared pair. Notice that the products are free-radicals.
For instance, the bond enthalpy, or bond-dissociation energy, for one of the C-H bonds in ethane (C2H6) is defined by the process:
The strength of bonds between different atoms varies across the periodic table and is well documented.
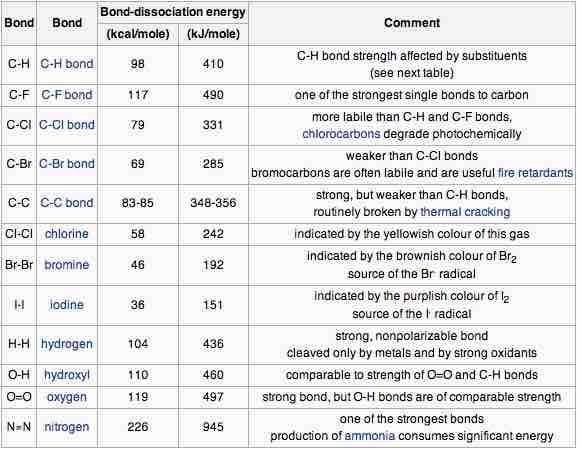
Bond dissociation energy
Bond dissociation energies for different element pairings are listed. It is evident that bond strength varies significantly for different combinations of elements in the periodic table.
Each bond in a molecule has its own bond dissociation energy, so a molecule with four bonds will require more energy to break the bonds than a molecule with one bond. As each successive bond is broken, the bond dissociation energy required for the other bonds changes slightly.