In chemistry, an ionic compound is a chemical compound in which ions are held together by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ionic compounds have high melting and boiling points, and they tend to be hard and brittle.
Ions can be single atoms, as the sodium and chlorine in common table salt (sodium chloride), or more complex (polyatomic) groups such as the carbonate in calcium carbonate. But to be considered an ion, they must carry a positive or negative charge. Thus, in an ionic bond, one 'bonder' must have a positive charge and the other a negative one. By sticking to each other, they resolve, or partially resolve, their separate charge imbalances. Positive to positive and negative to negative ionic bonds do not occur.
Most cations and anions can combine to form solid compounds that are usually known as salts. The one overriding requirement is that the resulting compound must be electrically neutral: therefore the ions Ca2+ and Br– combine only in a 1:2 ratio to form calcium bromide, CaBr2. Because no other simpler formula is possible, there is no need to name it "calcium dibromide." CaBr2 can be named using either the Stock method or the older, classic way of naming.
For example, CuCl2 indicates a molecule where one Cu2+ cation associates with two Cl- anions to form a neutral compound. Its systematic name is copper (II) chloride, where copper's oxidation number is indicated in parentheses. Its older name is cupric chloride.
The Stock Method of Naming
An ionic compound is named first by its cation and then by its anion. The cation has the same name as its element. For example, K+1 is called the potassium ion, just as K is called the potassium atom. The anion is named by taking the elemental name, removing the ending, and adding "-ide." For example, F-1 is called fluoride, for the elemental name, fluorine. The "-ine" was removed and replaced with "-ide." To name a compound, the cation name and the anion named are added together. For example, NaF is also known as sodium fluoride.
If either the cation or the anion was a polyatomic ion, the polyatomic ion name is used in the name of the overall compound. The polyatomic ion name stays the same. For example, Ca(NO3)2 is called calcium nitrate.
For cations that take on multiple charges (typically transition metals), the charge is written using Roman numerals in parentheses immediately following the element name. For example, Cu(NO3)2 is copper (II) nitrate, because the charge of two nitrate ions (NO3−1) is 2(-1) = -2. Since the net charge of the ionic compound must be zero, the Cu ion has a 2+ charge. This compound is therefore, copper (II) nitrate. The Roman numerals in fact show the oxidation number, but in simple ionic compounds this will always be the same as the metal's ionic charge.
The Old, Classic, or Common Way of Naming
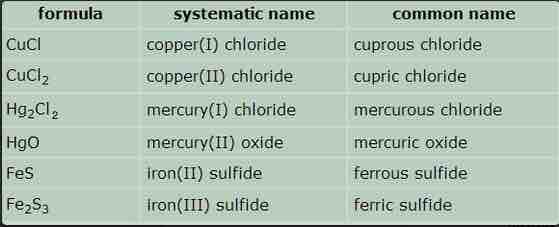
Names of some ionic compounds
Common, or trivial, names of compounds are sometimes used in informal conversations between chemists, especially older chemists. Systematic names are formal names that are always used in print.
Since some metallic elements form cations that have different positive charges, the names of ionic compounds derived from these elements must contain some indication of the cation charge. The older method uses the suffixes -ous and -ic to denote the lower and higher charges, respectively. In the cases of iron and copper, the Latin names of the elements are used (ferrous/ferric, cuprous/cupric). This system is still used, although it has been officially supplanted by the more precise, if slightly cumbersome, Stock system. In both systems, the name of the anion ends in -ide.