Section 5
Solution Concentration
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
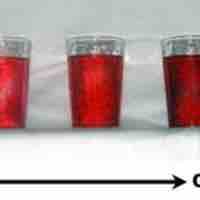
Dilutions of Solutions
Diluting a solution involves adding additional solvent to decrease the solution's concentration.
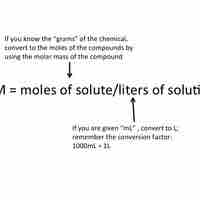
Using Molarity in Calculations of Solutions
Molarity is a unit of concentration; it is equal to moles of solute divided by the total volume of the solution in liters.

Solution Stoichiometry
Stoichiometry can be used to calculate the quantitative relationships between species in aqueous solution.