Concept
Version 16
Created by Boundless
sp2 Hybridization
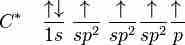
sp2 hybridization in ethene
In sp^2 hybridization, the 2s orbital mixes with only two of the three available 2p orbitals, forming a total of three sp^2 orbitals with one p-orbital remaining.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Inorganic Chemistry/Chemical Bonding/Orbital hybridization."
http://en.wikibooks.org/wiki/Inorganic_Chemistry/Chemical_Bonding/Orbital_hybridization%23sp_hybrids
Wikibooks
CC BY-SA 3.0.