Qualitative Analysis
Classical qualitative inorganic analysis is a method of analytical chemistry that seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution. The solution is treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, solid forming, and other visible changes.
Solubility Product Principle and Qualitative Analysis
Solubility-product constants can be used to devise methods for separating ions in a solution by selective precipitation. Selective precipitation is used to form a solid with one of the ions in solution without disturbing the other ions. You can continue this method to effectively separate all of the ions in a solution. The entire traditional qualitative-analysis scheme is based on the use of these equilibrium constants to determine the correct precipitating ions and the correct strategy.
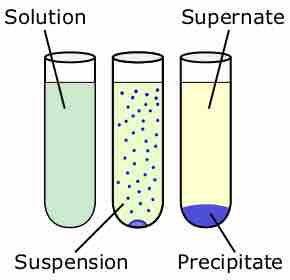
Chemical precipitation
Precipitation is the formation of a solid in a solution or inside another solid during a chemical reaction or by diffusion in a solid. Precipitation is used in qualitative chemical analysis.
Qualitative Analysis of Cations
Cations are usually classified into six groups. Each group has a common reagent that can be used to separate them from the solution. Because cationic analysis is based on the solubility products of the ions, meaningful results can be obtained only if separation is performed in a specified sequence. This is due to the fact that some ions of one group may also react with the reagent of another group. For example, both Ba2+ and Sr2+ will react with the SO42- ion to form a solid. Therefore, mathematical calculations should be done before choosing the SO42- ion for selective precipitation in a solution that may contain both Ba2+ and Sr2+.
Example:
A solution is 0.010M in barium chloride (BaCl2) and 0.020M in strontium chloride (SrCl2). Can either Ba2+ or Sr2+ be precipitated selectively with concentrated sodium sulfate (Na2SO4) solution? Which ion will precipitate first? (For simplicity, assume that the Na2SO4 solution is so concentrated that the volume change in the Ba-Sr solution can be neglected.)
Solution:
The barium sulfate solubility is given by:
Ksp = [Ba2+][SO42-] = 1.5 x 10-9
With 0.010
[SO42-] =
Strontium sulfate will precipitate when the sulfate concentration is:
This signifies that as the sulfate ion (SO42-) is added to solution and its concentration increases, barium will precipitate first.