In nuclear physics, stability of an atom's nucleus depends on the number of protons and neutrons it contains. The protons, which are both positively charged, repel one another through electrostatic force. This force is offset by the nuclear force, which attracts protons and neutrons.
To an extent, nuclei become more stable with increasing neutron number. This is because, for any constant number of protons, the difference between nuclear force and electrostatic repulsion of protons increases with increasing neutron count. However, if neutron count surpasses an ideal ratio, a nucleus becomes unstable and can undergo radioactive decay.
Among elements of atomic number 1-82, only two (technetium and promethium) lack at least one isotope considered to be stable. Only 90 isotopes in this region are believed to be perfectly stable, while 163 more are understood to be theoretically unstable but have never been observed to decay. Technetium and promethium, as well as elements of number 83 and above, have only isotopes that will decay over time.
Magic Numbers
Like electrons, nucleons can be arranged in shells that are most stable in certain numbers. These are called "magic numbers," and include 2, 8, 20, 28, 50, 82 and 126. Higher magic numbers have been calculated but were later dismissed, as it was found they were based on a spherical model of nuclei that does not apply to higher nuclei counts.
The term "double magic" is used to describe nuclei in which the numbers of protons and neutrons are both magic numbers. An example is nickel-48, which has 28 protons and 20 neutrons, both of which are magic numbers.
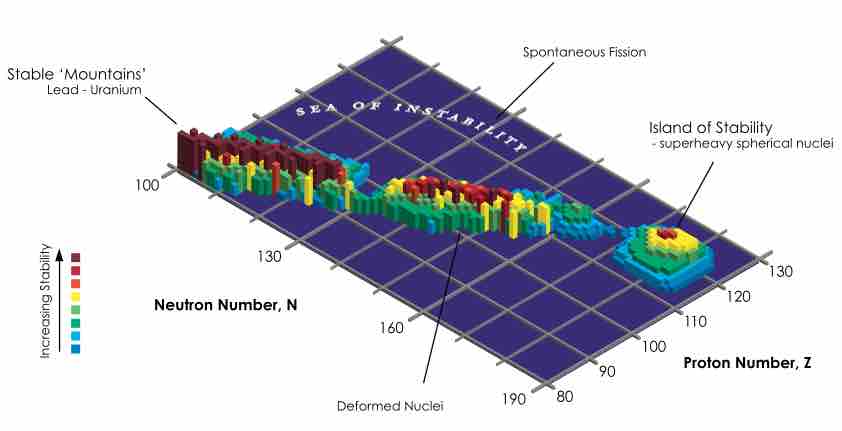
Table of Nuclear Stability
Stability of isotopes is shown as a function of proton and neutron numbers.