Concept
Version 6
Created by Boundless
Changes in Concentration
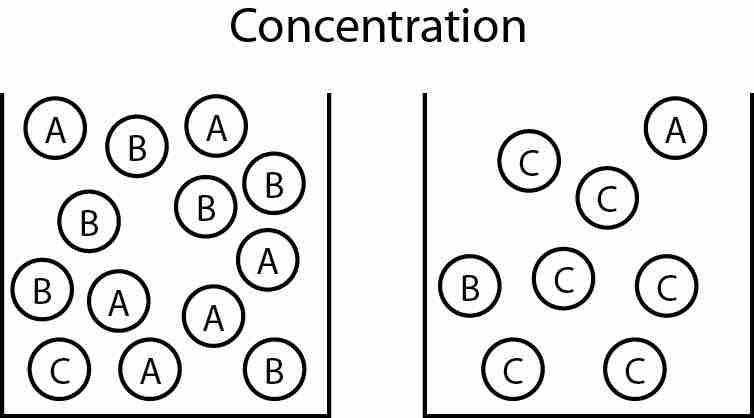
Changes in Concentration
In the course of a reaction, the total number of molecules can change. For instance, multiple molecules of the reactants (A and B) must be used to form a single molecule of product (C).
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Concentration Changes due to Reaction."
http://commons.wikimedia.org/wiki/File:Concentration_Changes_due_to_Reaction.png
Wikimedia
CC BY-SA.