Regulatory Proteins
The binding of the myosin heads to the muscle actin is a highly-regulated process. When a muscle is in a resting state, actin and myosin are separated. To keep actin from binding to the active site on myosin, regulatory proteins block the molecular binding sites. Tropomyosin blocks myosin binding sites on actin molecules, preventing cross-bridge formation, which prevents contraction in a muscle without nervous input. The protein complex troponin binds to tropomyosin, helping to position it on the actin molecule.
Regulation of Troponin and Tropomyosin
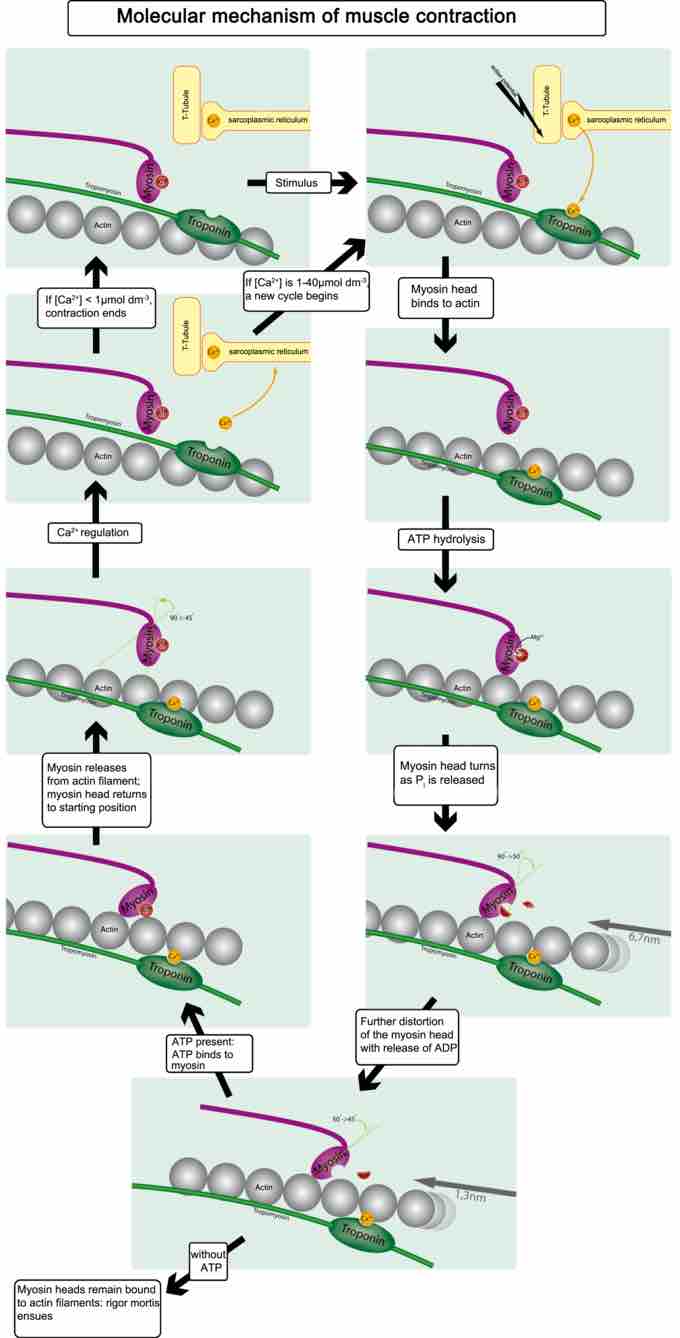
To enable muscle contraction, tropomyosin must change conformation and uncover the myosin-binding site on an actin molecule, thereby allowing cross-bridge formation. Troponin, which regulates the tropomyosin, is activated by calcium, which is kept at extremely low concentrations in the sarcoplasm. If present, calcium ions bind to troponin, causing conformational changes in troponin that allow tropomyosin to move away from the myosin-binding sites on actin. Once the tropomyosin is removed, a cross-bridge can form between actin and myosin, triggering contraction. Cross-bridge cycling continues until Ca2+ ions and ATP are no longer available; tropomyosin again covers the binding sites on actin .
Muscle contraction
Calcium remains in the sarcoplasmic reticulum until released by a stimulus. Calcium then binds to troponin, causing the troponin to change shape and remove the tropomyosin from the binding sites. Cross-bridge cling continues until the calcium ions and ATP are no longer available.
Calcium-Induced Calcium Release
The concentration of calcium within muscle cells is controlled by the sarcoplasmic reticulum, a unique form of endoplasmic reticulum in the sarcoplasm. Muscle contraction ends when calcium ions are pumped back into the sarcoplasmic reticulum, allowing the muscle cell to relax. During stimulation of the muscle cell, the motor neuron releases the neurotransmitter acetylcholine, which then binds to a post-synaptic nicotinic acetylcholine receptor.
A change in the receptor conformation causes an action potential, activating voltage-gated L-type calcium channels, which are present in the plasma membrane. The inward flow of calcium from the L-type calcium channels activates ryanodine receptors to release calcium ions from the sarcoplasmic reticulum. This mechanism is called calcium-induced calcium release (CICR). It is not understood whether the physical opening of the L-type calcium channels or the presence of calcium causes the ryanodine receptors to open. The outflow of calcium allows the myosin heads access to the actin cross-bridge binding sites, permitting muscle contraction.