Matter and Elements
Matter comprises all of the physical objects in the universe, those that take up space and have mass. All matter is composed of atoms of one or more elements, pure substances with specific chemical and physical properties. There are 98 elements that naturally occur on earth, yet living systems use a relatively small number of these. Living creatures are composed mainly of just four elements: carbon, hydrogen, oxygen, and nitrogen (often remembered by the acronym CHON). As elements are bonded together they form compounds that often have new emergent properties that are different from the properties of the individual elements. Life is an example of an emergent property that arises from the specific collection of molecules found in cells.
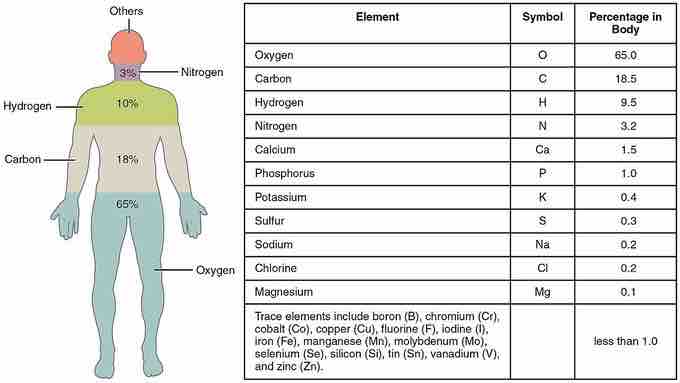
Elements of the human body arranged by percent of total mass
There are 25 elements believed to play an active role in human health. Carbon, hydrogen, oxygen, and nitrogen make up approximately 96% of the mass in a human body.
The Periodic Table
The different elements are organized and displayed in the periodic table. Devised by Russian chemist Dmitri Mendeleev (1834–1907) in 1869, the table groups elements that, although unique, share certain chemical properties with other elements. In the periodic table the elements are organized and displayed according to their atomic number and are arranged in a series of rows (periods) and columns (groups) based on shared chemical and physical properties. If you look at a periodic table, you will see the groups numbered at the top of each column from left to right starting with 1 and ending with 18. In addition to providing the atomic number for each element, the periodic table also displays the element's atomic mass. Looking at carbon, for example, its symbol (C) and name appear, as well as its atomic number of six (in the upper left-hand corner) and its atomic mass of 12.11.
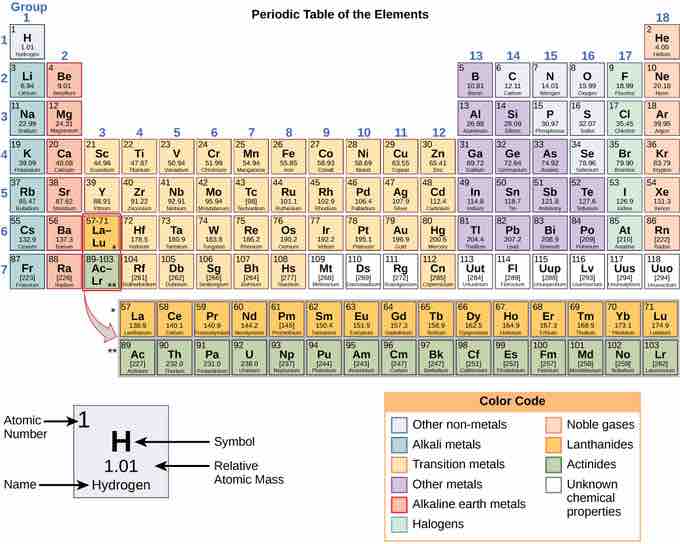
The periodic table
The periodic table shows the atomic mass and atomic number of each element. The atomic number appears above the symbol for the element and the approximate atomic mass appears below it.
The arrangement of the periodic table allows the elements to be grouped according to their chemical properties. Within the main group elements ( Groups 1-2, 13-18), there are some general trends that we can observe. The further down a given group, the elements have an increased metallic character: they are good conductors of both heat and electricity, solids at room temperature, and shiny in appearance. Moving from left to right across a period, the elements have greater non-metallic character. These elements are insulators, poor heat conductors, and can exist in different phases at room temperature (brittle solid, liquid, or gas). The elements at the boundary between the metallic elements (grey elements) and nonmetal elements (green elements) are metalloid in character (pink elements). They have low electrical conductivity that increases with temperature. They also share properties with both the metals and the nonmetals.
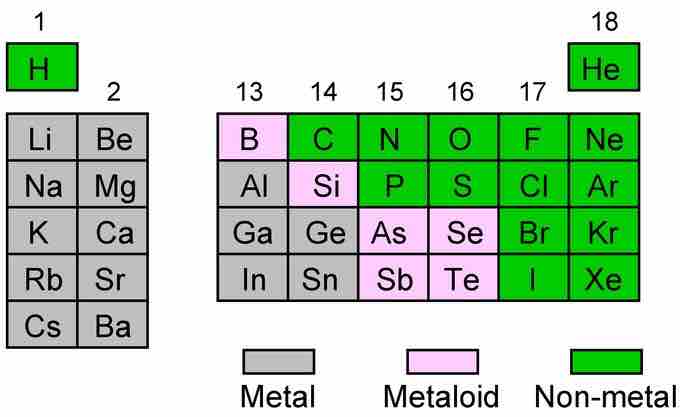
The main group elements
Within the p-block at the boundary between the metallic elements (grey elements) and nonmetal elements (green elements) there is positioned boron and silicon that are metalloid in character (pink elements), i.e., they have low electrical conductivity that increases with temperature.
Today, the periodic table continues to expand as heavier and heavier elements are synthesized in laboratories. These large elements are extremely unstable and, as such, are very difficult to detect; but their continued creation is an ongoing challenge undertaken by scientists around the world.