Structure of Steroid Molecules
Unlike phospholipids and fats, steroids have a fused ring structure. Although they do not resemble the other lipids, they are grouped with them because they are also hydrophobic and insoluble in water. All steroids have four linked carbon rings, and many of them, like cholesterol, have a short tail. Many steroids also have the –OH functional group, and these steroids are classified as alcohols called sterols.
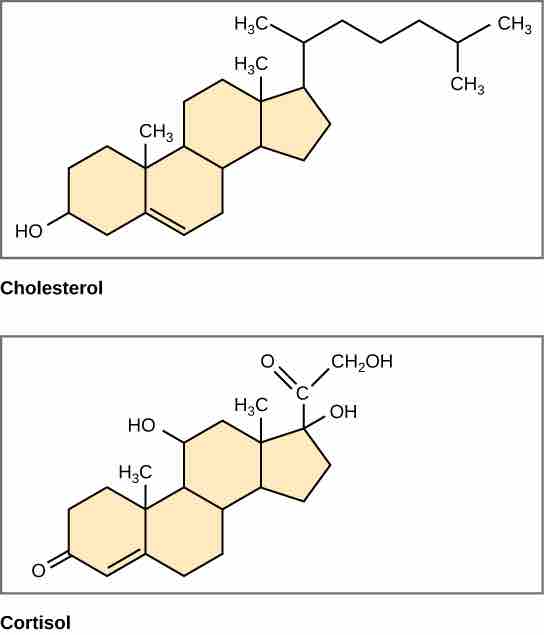
Steroid Structures
Steroids, such as cholesterol and cortisol, are composed of four fused hydrocarbon rings.
Cholesterol
Cholesterol is the most common steroid and is mainly synthesized in the liver; it is the precursor to vitamin D. Cholesterol is also a precursor to many important steroid hormones like estrogen, testosterone, and progesterone, which are secreted by the gonads and endocrine glands. Therefore, steroids play very important roles in the body's reproductive system. Cholesterol also plays a role in synthesizing the steroid hormones aldosterone, which is used for osmoregulation, and cortisol, which plays a role in metabolism.
Cholesterol is also the precursor to bile salts, which help in the emulsification of fats and their absorption by cells. It is a component of the plasma membrane of animal cells and the phospholipid bilayer. Being the outermost structure in animal cells, the plasma membrane is responsible for the transport of materials and cellular recognition; and it is involved in cell-to-cell communication. Thus, steroids also play an important role in the structure and function of membranes.
It has also been discovered that steroids can be active in the brain where they affect the nervous system, These neurosteroids alter electrical activity in the brain. They can either activate or tone down receptors that communicate messages from neurotransmitters. Since these neurosteroids can tone down receptors and decrease brain activity, steroids are often used in anesthetic medicines.