Caesium fluoride
About this schools Wikipedia selection
This selection is made for schools by a children's charity read more. SOS Children works in 45 African countries; can you help a child in Africa?
| Caesium fluoride | |
|---|---|
 |
|
 |
|
|
Caesium fluoride |
|
|
Other names
Cesium fluoride |
|
| Identifiers | |
| CAS number | 13400-13-0 |
| ChemSpider | 24179 |
| RTECS number | FK9650000 |
| Jmol-3D images | Image 1 |
|
SMILES
|
|
|
InChI
|
|
| Properties | |
| Molecular formula | CsF |
| Molar mass | 151.90 g/mol |
| Appearance | white crystalline solid |
| Density | 4.115 g/cm3 |
| Melting point |
682 °C (955 K) |
| Boiling point |
1251 °C (1524 K) |
| Solubility in water | 367 g/100 ml (18 °C) |
| Structure | |
| Crystal structure | cubic, cF8 |
| Space group | Fm3m, No. 225 |
| Coordination geometry |
Octahedral |
| Dipole moment | 7.9 D |
| Hazards | |
| MSDS | External MSDS |
| EU Index | Not listed |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions | Caesium chloride Caesium bromide Caesium iodide |
| Other cations | Lithium fluoride Sodium fluoride Potassium fluoride Rubidium fluoride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Caesium fluoride (cesium fluoride in North America), is an inorganic compound usually encountered as a hygroscopic white solid. It is more soluble and more readily dissociated than sodium fluoride or potassium fluoride. It is available in anhydrous form, and if water has been absorbed it is easy to dry by heating at 100 °C for two hours in vacuo. Like all soluble fluorides, it is mildly basic. An interesting fact about this compound is that it is the most ionic compound. Caesium has the lowest electronegativity (other than francium, an unstable extremely rare radioactive element), and fluorine has the highest electronegativity.
Synthesis and properties
Caesium fluoride is prepared by the action of hydrofluoric acid on caesium hydroxide or caesium carbonate, followed by removal of water.
Caesium fluoride reacts usually as a source of fluoride ion, F-. It therefore undergoes all of the usual reactions associated with soluble fluorides, for example:
- 2 CsF + CaCl2 → 2 CsCl + CaF2
Crystal structure
Caesium fluoride has the halite structure, which means that the Cs+ and F- pack in a cubic closest packed array as do Na+ and Cl- in sodium chloride. Caesium cations are larger than fluoride anions, whereas in the lithium, sodium, potassium, and rubidium halides, the cations are smaller than the anion.
Applications
In organic synthesis
Being highly dissociated it is a more reactive source of fluoride than related salts. CsF is less hygroscopic alternative to tetra-n-butylammonium fluoride (TBAF) and TAS-fluoride (TASF) when anhydrous "naked" fluoride ion is needed.
As a base
As with other soluble fluorides, CsF is moderately basic, because HF is a weak acid. The low nucleophilicity of fluoride means it can be a useful base in organic chemistry.Caesium fluoride is a useful base in organic chemistry, due the fact that fluoride ion is a relatively poor nucleophile. CsF gives higher yields in Knoevenagel condensation reactions than KF or NaF.
Formation of C-F bonds
Caesium fluoride is also a popular source of fluoride in organofluorine chemistry. For example, CsF reacts with hexafluoroacetone to form a caesium perfluoroalkoxide salt, which is stable up to 60 °C, unlike the corresponding sodium or potassium salt. It will convert electron-deficient aryl chlorides to aryl fluorides ( halex reaction).
Deprotection agent
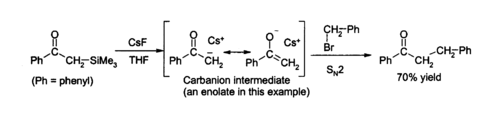
Due to the strength of the Si–F bond, fluoride ion is useful for desilylation reactions (removal of Si groups) in organic chemistry; caesium fluoride is an excellent source of anhydrous fluoride for such reactions. Removal of silicon groups ( desilylation) is a major application for CsF in the laboratory, as its anhydrous nature allows clean formation of water-sensitive intermediates. Solutions of caesium fluoride in THF or DMF attack a wide variety of organosilicon compounds to produce an organosilicon fluoride and a carbanion, which can then react with electrophiles, for example:
Desilylation is also useful for the removal of silyl protecting groups.
Other uses
Single crystals of the salt are transparent into the deep infrared. For this reason it is sometimes used as the windows of cells used for infrared spectroscopy.
Precautions
Like other soluble fluorides, CsF is moderately toxic. Contact with acid should be avoided, as this forms highly toxic/corrosive hydrofluoric acid. Caesium ion (Cs+), or caesium chloride, is generally not considered toxic.