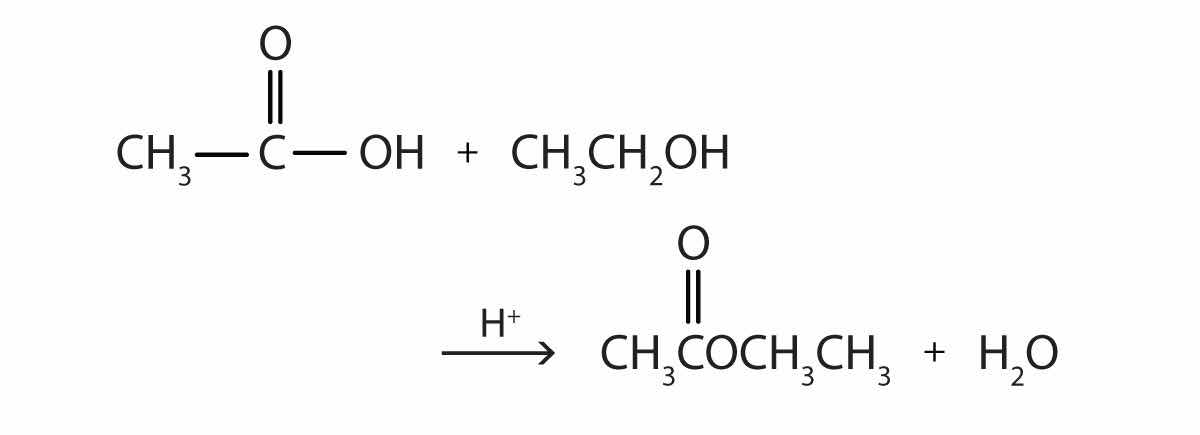
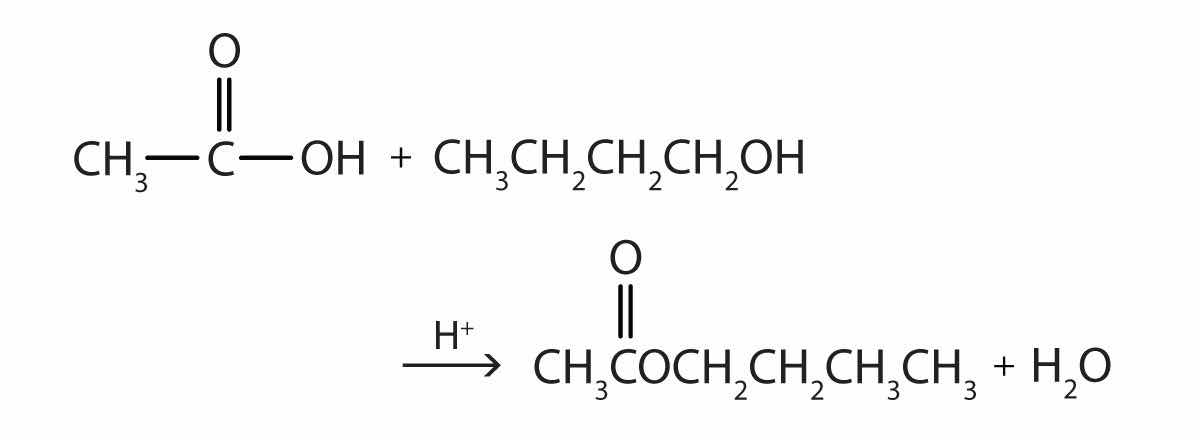
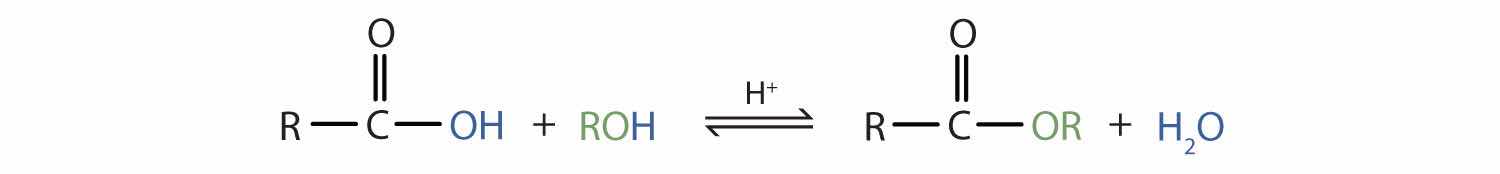
Some esters can be prepared by esterificationThe formation of an ester from a carboxylic acid and an alcohol., a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water:
The reaction is reversible. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
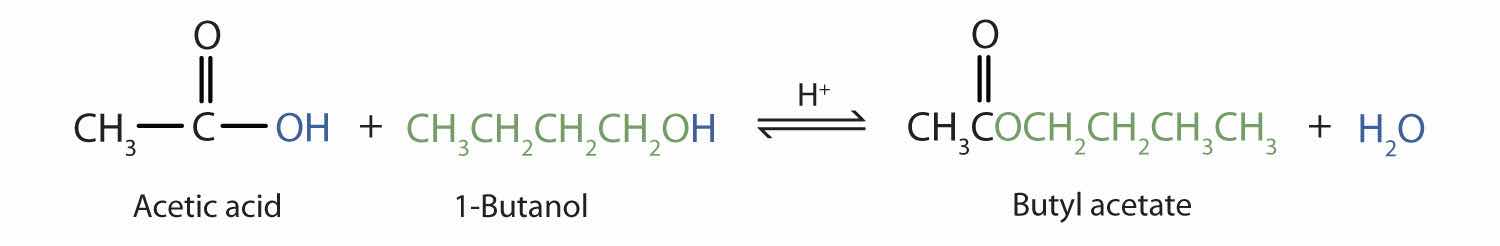
A commercially important esterification reaction is condensation polymerization, in which a reaction occurs between a dicarboxylic acid and a dihydric alcohol (diol), with the elimination of water. Such a reaction yields an ester that contains a free (unreacted) carboxyl group at one end and a free alcohol group at the other end. Further condensation reactions then occur, producing polyester polymers.
The most important polyester, polyethylene terephthalate (PET), is made from terephthalic acid and ethylene glycol monomers:
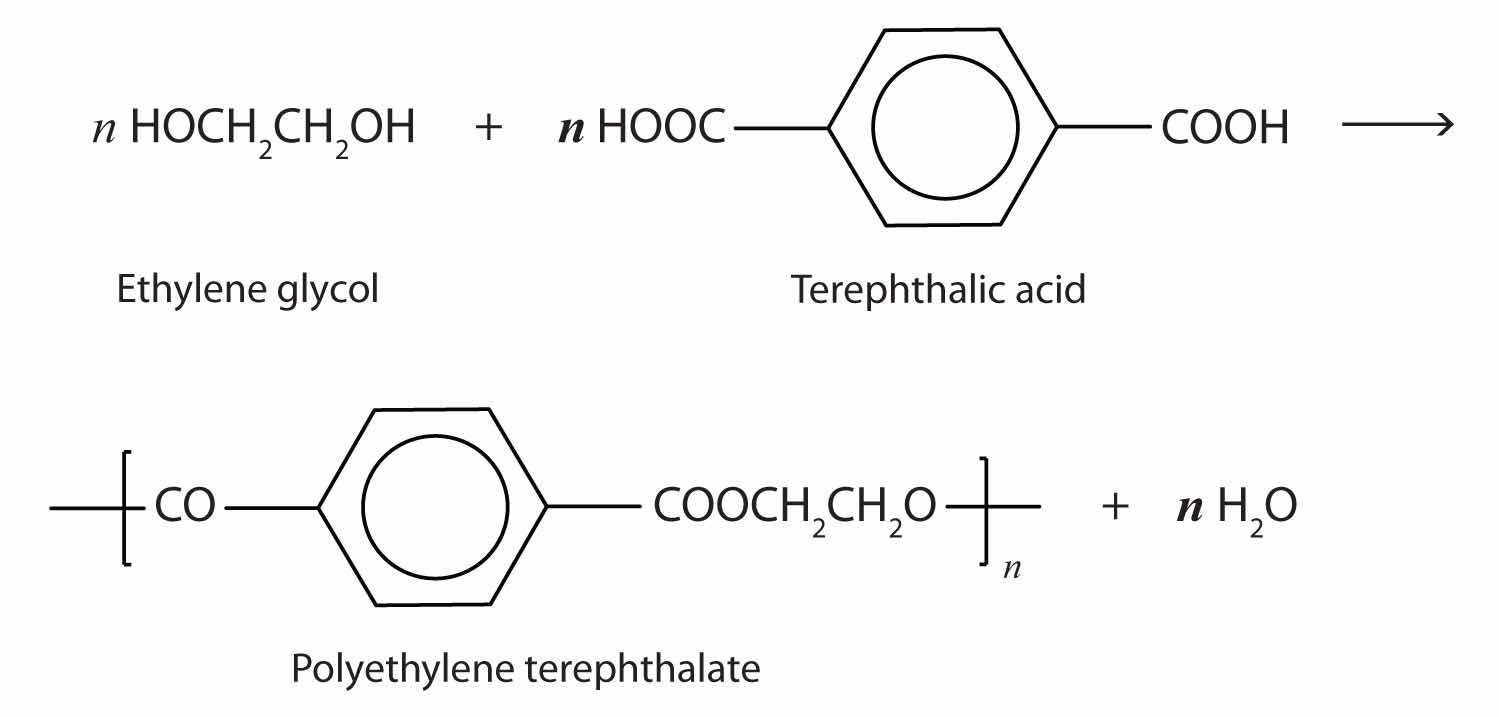
Polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda pop and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes. Synthetic arteries can be made from PET, polytetrafluoroethylene, and other polymers.
From what carboxylic acid and what alcohol can the ester isopropyl nonanoate be made?
From what carboxylic acid and what alcohol can the ester cyclobutyl butyrate be made?
nonanoic acid and isopropyl alcohol
butyric acid and cyclobutyl alcohol
Write the equation for the reaction of acetic acid with each compound.
Write the equation for the reaction of benzoic acid with each compound.