Wetting
From Wikipedia, the free encyclopedia
Wetting is the contact between a fluid and a surface, when the two are brought into contact. When a liquid has a high surface tension (strong internal bonds), it will form a droplet, whereas a liquid with low surface tension will spread out over a greater area (bonding to the surface). On the other hand, if a surface has a high surface energy (or surface tension), a drop will spread, or wet, the surface. If the surface has a low surface energy, a droplet will form. This phenomenon is a result of the minimization of interfacial energy. If the surface has a high energy, it will want to be covered with a liquid because this interface will lower its energy, and so on.
The primary measurement to determine wettability is a contact angle measurement. This measures the angle between the surface and the surface of a liquid droplet on the surface. For example, a droplet would have a high contact angle, but a liquid spread on the surface would have a small one. The contact angle  and the surface energies of the materials involved are related by the Young–Dupré equation
and the surface energies of the materials involved are related by the Young–Dupré equation
 [1]
[1]
where 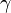 is the surface tension between two substances and S, V, and L correspond to the solid, vapor, and liquid substances in a contact angle experiment respectively.
is the surface tension between two substances and S, V, and L correspond to the solid, vapor, and liquid substances in a contact angle experiment respectively.
A contact angle of 90° or greater generally characterizes a surface as not-wettable, and one less than 90° means that the surface is wettable. In the context of water, a wettable surface may also be termed hydrophilic and a non-wettable surface hydrophobic. Superhydrophobic surfaces have contact angles greater than 150°, showing almost no contact between the liquid drop and the surface. This is sometimes referred to as the "Lotus effect". This characteristic of spreading out over a greater area is sometimes called 'wetting action' when discussing solders and soldering.
Wetting is often an important factor in the bonding (adherence) of two materials. It is also the basis for capillary action, the ability of a narrow tube to draw a liquid, even against the force of gravity.
The shape of a drop is roughly a spherical cap.
