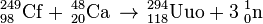Ununoctium
From Wikipedia, the free encyclopedia
| |||||||||||||
| General | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | ununoctium, Uuo, 118 | ||||||||||||
| Chemical series | noble gases | ||||||||||||
| Group, Period, Block | 18, 7, p | ||||||||||||
| Appearance | unknown, probably colorless | ||||||||||||
| Standard atomic weight | predicted, (314) g·mol−1 | ||||||||||||
| Electron configuration | perhaps [Rn] 7s2 5f14 6d10 7p6 (guess based on radon) | ||||||||||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8 | ||||||||||||
| Phase | presumably a gas | ||||||||||||
| CAS registry number | 54144-19-3 | ||||||||||||
| Selected isotopes | |||||||||||||
| |||||||||||||
| References | |||||||||||||
|
Ununoctium (IPA pronunciation: /ˌjuːnəˈnɒktiəm/ [1]) is the temporary IUPAC name for the superheavy element having atomic number of 118, currently the highest atomic number assigned to a reputedly discovered element. It has the temporary IUPAC element symbol Uuo. It can also be called eka-radon and only three atoms of this element have been detected. [2] The name ununoctium is a systematic element name, used as a placeholder until the IUPAC decides on a name.
[edit] Discovery
On October 16, 2006, researchers working at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, announced in Physical Review C that they had indirectly detected a total of three nuclei of ununoctium-294 (one in 2002 and two more in 2005) produced via collisions of californium-249 atoms and calcium-48 ions [3][4]:
Because of the very small fusion reaction probability (the fusion cross section is 0.5 pb = 5×10−41 m2) more than 4×1019 calcium ions had to be shot at the californium to have only three fusion reactions.
The research team consisted of workers from JINR and the Lawrence Livermore National Laboratory in California, USA. The decay products of three atoms of ununoctium, not the atoms themselves, were observed in Dubna. A half-life of 0.89 ms was observed: 294Uuo decays into 290Uuh by alpha decay. Since there were only three nuclei, the half-life was derived from observed lifetimes and has a large uncertainty: 0.89-0.31+1.07 ms.
The identification of the 294Uuo nuclei was verified by separately creating the putative daughter nucleus 290Uuh by means of a bombardment of 245Cm with 48Ca ions,
and checking that the 290Uuh decay matched the decay chain of the 294Uuo nuclei.
The daughter nucleus 290Uuh is very unstable, decaying with a half-life of 14 milliseconds into 286Uuq, which may undergo spontaneous fission or undergo alpha decay into 282Uub, which will undergo spontaneous fission. [5]
[edit] History
In 1999, researchers at Lawrence Berkeley National Laboratory had announced the discovery of elements 116 and 118, in a paper published in Physical Review Letters. [6]
The researchers claimed to have performed the reaction:
The following year, they published a retraction after other researchers were unable to duplicate the results.[7] In June 2002, the director of the lab announced that the original claim of the discovery of these two elements had been based on data fabricated by principal author Victor Ninov.
The American group had intended to name it ghiorsium after Albert Ghiorso before having to retract their claim.