Standard conditions for temperature and pressure
From Wikipedia, the free encyclopedia
In chemistry and other sciences, STP or standard temperature and pressure is a standard set of conditions for experimental measurements, to enable comparisons to be made between sets of data. Internationally, the current STP defined by the IUPAC (International Union of Pure and Applied Chemistry) is an absolute pressure of 100.00 kPa (1 bar) and a temperature of 273.15 K (0 °C).[1] Other organizations have established a variety of alternative definitions for the standard reference conditions of temperature and pressure, such as the SATP amongst others.
In industry and commerce, it is necessary to define the standard reference conditions of temperature and pressure when expressing a gas volume or a volumetric flow rate because the volume of a gas varies with the temperature and pressure of the gas. The available data on the various definitions of standard reference conditions clearly indicates that the IUPAC's STP is not a universally accepted definition of the standard conditions of temperature and pressure. For that reason, simply stating that a gas flow rate is 10,000 m³/h (i.e. cubic meters per hour) at "standard conditions" or at "STP" has no meaning unless the reference conditions that were applied are clearly stated.
In aeronautics and fluid dynamics the term "International Standard Atmosphere" is often used to denote the variation of the principal thermodynamic variables (pressure, temperature, density, etc.) of the atmosphere with altitude at mid latitudes.
Contents
|
[edit] Definitions used in the past
For a great many years, most engineers, chemists, physicists and other scientists using the metric system of units defined the standard reference conditions of temperature and pressure for expressing gas volumes as being 0 °C (273.15 K) and 101.325 kPa (i.e., 1 atmosphere of absolute pressure). During those same years, the most commonly used standard reference conditions for people using the Imperial or customary USA system of units was 60 °F (520 °R) and 14.696 psia (i.e., 1 atmosphere of absolute pressure) because it was almost universally used by the oil and gas industries worldwide.
The above two definitions are no longer the most commonly used definitions in either the metric, Imperial or the customary USA system of units. Some of the many different definitions currently in use are presented in the next section.
It was also common in the past, when using the metric system of units, to refer to a Normal Cubic Meter (Nm³) and to define it as being at 0 °C (273.15 K) and 101.325 kPa (i.e. 1 atmosphere of absolute pressure). As shown in the following section, that notation is no longer appropriate unless the specific reference conditions are explicitly stated, since there are currently many different metric system definitions of what constitutes standard reference conditions.
In the same manner, it is also no longer appropriate to refer to a standard cubic foot (scf) unless the specific reference conditions are explicitly stated, again because there are currently many different definitions of the standard reference condition in both the Imperial and the customary U.S. systems of units. In particular, OPEC and a majority of the natural gas industry in North America have adopted 60 °F and 14.73 psia as their standard reference conditions for expressing natural gas volumes and flow rates (rather than the 60 °F and 14.696 psia commonly used previously).
[edit] Definitions in current use
There are a great many different definitions of the standard reference conditions currently being used. Table 1 presents twelve such variations of standard condition definitions - and there are quite a few others as well.
As shown in the table, the IUPAC (International Union of Pure and Applied Chemistry) currently defines standard reference conditions as being 0 °C and 1 bar (i.e., 100 kPa) of absolute pressure rather than the 1 atmosphere (i.e. 101.325 kPa) of absolute pressure used in the past. In fact, the IUPAC's current definition has been in existence since 1982.[2]
As further shown in the table, the oil and gas industries have to a large extent changed from their past usage of 60 °F and 14.696 psia to their current usage of 60 °F and 14.73 psia. This is especially true of the natural gas industry in North America.
For the SATP used in presenting chemical thermodynamic properties (such as those published by the National Bureau of Standards as included in Table 1) that the pressure is standardized at 1 bar (100 kPa) but the temperature may vary and needs to be specified separately.
It should also be noted that the International Organization for Standardization (ISO), the United States Environmental Protection Agency (EPA) and National Institute of Standards and Technology (NIST) each have more than one definition of standard reference conditions in their various standards and regulations.
The table makes it quite obvious that it is absolutely necessary to clearly state the temperature and pressure reference conditions whenever expressing a gas volume or gas volumetric flowrate. It is equally important to state whether the gas volume is expressed on a dry basis or a wet basis. As noted in the table, some of the current definitions of the reference conditions include a specification of the percent relative humidity (% RH).
| Temperature | Absolute pressure | Relative humidity | Publishing or establishing entity |
|---|---|---|---|
| °C | kPa | % RH | |
| 0 | 100.000 | IUPAC (present definition)[1] | |
| 0 | 101.325 | IUPAC (former definition),[1] NIST,[3] ISO 10780[4] | |
| 15 | 101.325 | 0 [4], [5] | ISA,[5] ISO 13443,[6] EEA,[7] EGIA[8] |
| 20 | 101.325 | EPA,[9] NIST[10] | |
| 25 | 101.325 | EPA[11] | |
| 25 | 100.000 | SATP[12] | |
| 20 | 100.000 | 0 | CAGI[13] |
| 15 | 100.000 | SPE[14] | |
| °F | psia | % RH | |
| 60 | 14.696 | SPE,[14] OSHA,[15] SCAQMD[16] | |
| 60 | 14.73 | EGIA,[8] OPEC,[17] EIA[18] | |
| 59 | 14.503 | 78 | Army Standard Metro[19] |
| 59 | 14.696 | 60 | ISO 2314, ISO 3977-2[20] |
Notes:
- 101.325 kPa = 1 atmosphere = 1.01325 bar ≈ 14.696 psi
- 100.000 kPa = 1 bar ≈ 14.504 psi
- 14.503 psi ≈ 750 mmHg ≈ 100.0 kPa ≈ 1 bar
- 14.696 psi ≈ 1 atm = 101.325 kPa
- 14.73 psi ≈ 30 inHg ≈ 1.0156 bar ≈ 101.560 kPa
- All pressures are absolute pressures (not gauge pressures)
- 59 °F = 15 °C
- 60 °F ≈ 15.6 °C
- dry = 0 percent relative humidity = 0 % RH
The full names of the entities listed in Table 1:
- IUPAC: International Union of Pure and Applied Chemistry
- NIST: National Institute of Standards and Technology
- ISA: ICAO's International Standard Atmosphere
- ISO: International Organization for Standardization
- EEA: European Environment Agency
- EGIA: Electricity and Gas Inspection Act (of Canada)
- EPA: United States Environmental Protection Agency
- SATP: Standard Ambient Pressure and Temperature
- CAGI: Compressed Air and Gas Institute
- SPE: Society of Petroleum Engineers
- OSHA: U.S. Occupational Safety and Health Administration
- SCAQMD: California's South Coast Air Quality Management District
- OPEC: Organization of Petroleum Exporting Countries
- EIA: U.S. Energy Information Administration
- Std. Metro: U.S. Army's Standard Metro (used in ballistics)
[edit] Molar volume of a gas
It is equally as important to indicate the applicable reference conditions of temperature and pressure when stating the molar volume of a gas[21] as it is when expressing a gas volume or volumetric flow rate. Stating the molar volume of a gas without indicating the reference conditions of temperature and pressure has no meaning and it can cause much confusion.
The molar gas volumes can be calculated with an accuracy that is usually sufficient by using the universal gas law for ideal gases. The usual expression is:
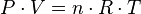
…which can be rearranged thus:
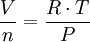
where (in SI metric units):
| P | = the gas absolute pressure, in Pa |
|---|---|
| n | = number of moles, in mol |
| V / n | = the gas molar volume, in m³/mol |
| T | = the gas absolute temperature, in K |
| R | = the universal gas law constant of 8.3145 m³·Pa/(mol·K) |
or where (in customary USA units):
| P | = the gas absolute pressure, in psia |
|---|---|
| n | = number of moles, in lbmol |
| V / n | = the gas molar volume, in ft³/lbmol |
| T | = the gas absolute temperature, in °R |
| R | = the universal gas law constant of 10.7316 ft³·psia/(lbmol·°R) |
The molar volume of any ideal gas may be calculated at various standard reference conditions as shown below:
- V / n = 8.3145 × 273.15 / 101.325 = 22.414 m³/kmol at 0 °C and 101.325 kPa absolute pressure
- V / n = 8.3145 × 273.15 / 100.000 = 22.711 m³/kmol at 0 °C and 100 kPa absolute pressure
- V / n = 10.7316 × 519.67 / 14.696 = 379.48 ft³/lbmol at 60 °F and 14.696 psia absolute pressure
- V / n = 10.7316 × 519.67 / 14.730 = 378.61 ft³/lbmol at 60 °F and 14.73 psia absolute pressure
The technical literature can be very confusing because many authors fail to explain whether they are using the universal gas law constant R which applies to any ideal gas or whether they are using the gas law constant Rs which only applies to a specific individual gas. The relationship between the two constants is Rs = R / M, where M is the molecular weight of the gas.
It may be of interest to note that the US Standard Atmosphere still uses 8.31432 m³·Pa/(mol·K) as the value of R for all calculations. (See Gas constant)
