Rate equation
From Wikipedia, the free encyclopedia
According to IUPAC Gold Book[1] the rate law or rate equation for a chemical reaction is an equation which links the reaction rate with concentrations or pressures of reactants and constant parameters (normally rate coefficients and partial reaction orders). To determine the rate equation for a particular system one combines the reaction rate with a mass balance for the system [2]. For a generic reaction A + B → C the simple rate equation (as opposed to the much more common complicated rate equations) is of the form:
In this equation, [X] expresses the concentration of a given X, usually in mol/litre and k(T) is known as the reaction rate coefficient or rate constant, although it is not really a constant, because it includes everything that affects reaction rate outside concentration: mainly temperature but also ionic strength, surface area of the adsorbent or light irradiation.
The exponents n and m are called reaction orders and depend on the reaction mechanism. The stoichiometric coefficients and reaction orders are very often equal, but only in one step reactions, molecularity (number of molecules or atoms actually colliding), stoichiometry and reaction order must be the same.
Complicated rate equations are not of the form above, and they can be a sum of terms like it or have quantities in the denominator (see further sections)
The rate equation is a differential equation, and it can be integrated in order to obtain an integrated rate equation that links concentrations of reactants or products with time.
If the concentration of one of the reactants remains constant (because it is a catalyst or it is in great excess with respect to the other reactants) its concentration can be excluded in the rate constant, obtaining a pseudo constant: if B is the reactant whose concentration is constant then r = k[A][B] = k'[A]. The second order rate equation has been reduced to a pseudo first order rate equation. This makes the treatment to obtain an integrated rate equation much easier.
Contents
|
[edit] Zero-order reactions
A zero-order reaction has a rate which is independent of the concentration of the reactant(s). Increasing the concentration of the reacting species will not speed up the rate of the reaction. Zero-order reactions are typically found when a material required for the reaction to proceed, such as a surface or a catalyst, is saturated by the reactants. The rate law for a zero-order reaction is
Where r is the reaction rate and k is the reaction rate coefficient, k has units of concentration/time. If, and only if, this zero-order reaction 1) occurs in a closed system, 2) there is no net build-up of intermediates and 3) there are no other reactions occurring, it can be shown by solving a Mass balance for the system that:
If this differential equation is integrated it gives an equation which is often called the integrated zero-order rate law
where ![\ [A]](http://upload.wikimedia.org/math/5/7/f/57f3827b89cf104f4bd4c0a65fd4cfd2.png) represents the concentration of the chemical of interest at a particular time and
represents the concentration of the chemical of interest at a particular time and ![\ [A]_0](http://upload.wikimedia.org/math/c/c/3/cc3b9d535081e84d36778034689277d7.png) represents the initial concentration.
represents the initial concentration.
A reaction is zero order if concentration data are plotted versus time and the result is a straight line. The slope of this resulting line is the zero order rate constant k.
The half-life of a reaction describes the time needed for half of the reactant to be depleted (same as the half-life involved in nuclear decay, which is a first-order reaction). For a zero-order reaction the half-life is given by
![\ t_ \frac{1}{2} = \frac{[A]_0}{2k}](http://upload.wikimedia.org/math/6/e/4/6e4b883bbbf7a967b488081d26589d73.png) .
.
- Example of a Zeroth-order reaction
- Reversed Haber process:

[edit] First-order reactions
A first-order reaction depends on the concentration of only one reactant (a unimolecular reaction). Other reactants can be present, but each will be zero-order. The rate law for a first-order reaction is
k is the first order rate constant that has units of 1/time
If, and only if, this first-order reaction 1) occurs in a closed system, 2) there is no net build-up of intermediates and 3) there are no other reactions occurring, it can be shown by solving a mass balance for the system that
where a is the stoichiometric coefficient of the species A.
The integrated first-order rate law is
A plot of ![\ -ln{ [A]}](http://upload.wikimedia.org/math/7/0/b/70b6d268b353879d04d0acfc1cf1fe76.png) vs. time
vs. time 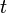 gives a straight line with a slope equal to the reaction rate constant. The half life of a first-order reaction can be determined using the equation
gives a straight line with a slope equal to the reaction rate constant. The half life of a first-order reaction can be determined using the equation 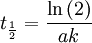 . Charactaristic of a first-order reaction is that all the half-lives are equal.
. Charactaristic of a first-order reaction is that all the half-lives are equal.
- Examples of First-order reactions
These are all first-order with respect to the reactant.
[edit] Second-order reactions
A second-order reaction depends on the concentrations of one second-order reactant, or two first-order reactants.
For a second order reaction, its reaction rate is given by:
![\ r = k[A]^2](http://upload.wikimedia.org/math/9/7/2/972faafa9cf37bb0dc3b5645fd9539f6.png) or
or ![\ r = k[A][B]](http://upload.wikimedia.org/math/5/8/3/583d2c3e5ce8e197a21080b80a284d84.png)
The integrated second-order rate laws are respectively
![\frac{1}{[A]} = kt + \frac{1}{[A]_0}](http://upload.wikimedia.org/math/e/f/5/ef537833c2216714739fda46f3cdb790.png) or
or
[A]0 and [B]0 must be different, in order to obtain that integrated equation.
The half-life equation for a second-order reaction dependent on one second-order reactant is ![\ t_ \frac{1}{2} = \frac{1}{k[A]_0}](http://upload.wikimedia.org/math/9/c/e/9ce65475b614802ec855072f11df0d99.png) . For a second-order reaction half-lives progressively double.
. For a second-order reaction half-lives progressively double.
Another way to present the above rate laws is to take the log of both sides: ![\ln{}r = \ln{}k + 2\ln\left[A\right]](http://upload.wikimedia.org/math/c/f/0/cf04309d2e7d76fc386ebfcf6877723f.png)
- Examples of a Second-order reaction
[edit] Pseudo first order
Measuring a second order reaction rate can be problematic: the concentrations of the two reactants must be followed simultaneously, which is more difficult; or measure one of them and calculate the other as a difference, which is less precise. A common solution for that problem is the pseudo first order approximation
If either [A] or [B] remain constant as the reaction proceeds, then the reaction can be considered pseudo first order because in fact it only depends on the concentration of one reactant. If for example [B] remains constant then:
![\ r = k[A][B] = k'[A]](http://upload.wikimedia.org/math/7/b/4/7b4a55d51c83a671365d244987234a56.png)
where k' = k[B]0 (k' or kobs with units s-1) and we have an expression identical to the first order expression above.
One way to obtain a pseudo first order reaction is to use a large excess of one of the reactants ([B]>>[A] would work for the previous example) so that, as the reaction progresses only a small amount of the reactant is consumed and its concentration can be considered to stay constant. By collecting k' for many reactions with different (but excess) concentrations of [B]; a plot of k' versus [B] gives k (the regular second order rate constant) as the slope.
[edit] Summary for reaction orders 0, 1, 2 and n
Reactions with order 3 are very rare, and extremely unlikely to occur. The known ones almost always involve dinitrogen pentoxide N2O5.[citation needed]
| Zero Order | First Order | Second Order | n-th Order | |
|---|---|---|---|---|
| Rate Law | ![-\frac{d[A]}{dt} = k](http://upload.wikimedia.org/math/b/3/0/b3002675ed0c609a10157d0c733543b0.png) | ![-\frac{d[A]}{dt} = k[A]](http://upload.wikimedia.org/math/e/8/0/e802dbfb8a252f2bf0c9729a4e6e66bb.png) | ![-\frac{d[A]}{dt} = k[A]^2](http://upload.wikimedia.org/math/5/8/c/58cd77b68b6d8db6df381fc176b25a1d.png) | ![-\frac{d[A]}{dt} = k [A]^n](http://upload.wikimedia.org/math/3/6/f/36f83d341091d001bf230ba93dc66181.png) |
| Integrated Rate Law | ![\ [A] = [A]_0 - kt](http://upload.wikimedia.org/math/8/6/f/86fcee80fab8997ba85af1ff815abeff.png) | ![\ [A] = [A]_0 e^{-kt}](http://upload.wikimedia.org/math/f/3/a/f3acb1952af0a546a0c3162b0c262341.png) | ![\frac{1}{[A]} = \frac{1}{[A]_0} + kt](http://upload.wikimedia.org/math/6/6/4/664842f28db997704b7ddbc61f6a7be8.png) | ![\frac{1}{[A]^{n-1}} = \frac{1}{{[A]_0}^{n-1}} + (n-1)kt](http://upload.wikimedia.org/math/e/d/4/ed46a209d9c598bad5186d11c6834122.png) [Except first order] |
Units of Rate Constant 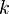 | 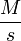 |  |  |  |
Linear Plot to determine 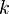 | ![[A] \ \mbox{vs.} \ t](http://upload.wikimedia.org/math/b/8/a/b8a126629e29133c39b43abc6d175bcd.png) | ![\ln ([A]) \ \mbox{vs.} \ t](http://upload.wikimedia.org/math/8/8/1/8813833afb591af2c5a05439c9f62355.png) | ![\frac{1}{[A]} \ \mbox{vs.} \ t](http://upload.wikimedia.org/math/4/d/7/4d793f30055b332220dc5c0cc98e394c.png) | ![\frac{1}{[A]^{n-1}} \ \mbox{vs.} \ t](http://upload.wikimedia.org/math/3/e/b/3ebf4ce732399ed4911ed113b04e5b8a.png) [Except first order] |
| Half-life | ![t_{1/2} = \frac{[A]_0}{2k}](http://upload.wikimedia.org/math/e/f/e/efe758e6239079a41ba9d39d5cc395c2.png) |  | ![t_{1/2} = \frac{1}{[A]_0 k}](http://upload.wikimedia.org/math/d/f/b/dfb4d205895976c529cf6f15bbde08d7.png) | ![t_{1/2} = \frac{2^{n-1}-1}{(n-1)k[A_0]^{n-1}}](http://upload.wikimedia.org/math/7/5/f/75f4655c5c7e737fd17fc3720f81bb06.png) [Except first order] |
[edit] Equilibrium reactions or opposed reactions
A pair of forward and reverse reactions may define an equilibrium process. For example A and B react into X and Y and vice versa (s, t, u and v are the stoichiometric coefficients):
sA + tB ⇌ uX + vY
The reaction rate expression for the above reactions (assuming they each are elementary) can be expressed as:
where: k1 is the rate coefficient for the reaction which consumes A and B; k2 is the rate coefficient for the backwards reaction, which consumes X and Y and produces A and B.
The constants k1 and k2 are related to the equilibrium coefficient for the reaction (K) by the following relationship (set r=0 in balance):
In a simple equilibrium between two species:
the constant K at equilibrium is expressed as:
When the concentration of A at equilibrium is that of the concentration at time 0 minus the conversion in moles
with x equal to the concentration of B at equilibrium
then it follows that
and
The reaction rate becomes:
which results in:
A plot of the negative natural logarithm of the concentration of A in time minus the concentration at equilibrium versus time t gives a straight line with slope kf + kr. By measurement of Ae and Be the values of K and the two reaction rate constants will be known [3].
When the equilibrium constant is close to unity and the reaction rates very fast for instance in conformational analysis of molecules, other methods are required for the determination of rate constants for instance by complete lineshape analysis in NMR spectroscopy.
[edit] Consecutive reactions
If the rate constants for the following reaction are k1 and k2;  , then the rate equation is:
, then the rate equation is:
For reactant A: ![\frac{d[A]}{dt} = -k_1 [A]](http://upload.wikimedia.org/math/8/6/e/86e24b62bbf0b6d0c81b6ff5143b8ab9.png)
For reactant B: ![\frac{d[B]}{dt} = k_1 [A] - k_2 [B]](http://upload.wikimedia.org/math/d/d/b/ddbf39f88d2aea78c65e997ae87434ae.png)
For product C: ![\frac{d[C]}{dt} = k_2 [B]](http://upload.wikimedia.org/math/3/9/b/39b9e2213ec97994c460c034d38c59ae.png)
These differential equations can be solved analytically and the integrated rate equations (supposing that initial concentrations of every substance except A are zero) are
![[A]=[A]_0 e^{-k_1 t}](http://upload.wikimedia.org/math/7/2/5/7256d2c1532cc5805adcedb96279b69a.png)
![[B]=[A]_0 \frac{k_1}{k_2 - k_1}\left ( e^{-k_1t}-e^{-k_2t} \right )](http://upload.wikimedia.org/math/c/0/0/c00ef757f86b0d88f333f3b279dfca07.png)
![[C] = \frac{[A]_0}{k_2-k_1} \left [ k_2 \left ( 1- e^{-k_1t} \right ) - k_1 \left (1- e^{-k_2t} \right ) \right ] \quad = [A]_0 \left (1 + \frac{k_1 e^{-k_2t}-k_2e^{-k_1t}}{k_2-k_1} \right )](http://upload.wikimedia.org/math/1/8/a/18a1c0f713c9f7485d1d81deb0de0517.png)
The steady state approximation leads to very similar results in an easier way.
[edit] Parallel or competitive reactions
When a substance reacts simultaneously to give two different products, a parallel or competitive reaction is said to have place.
- Two first order reactions:
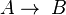 and
and 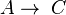 , with constants k1 and k2 and rate equations
, with constants k1 and k2 and rate equations ![-\frac{d[A]}{dt}=(k_1+k_2)[A]](http://upload.wikimedia.org/math/d/0/4/d04394a9bfcd42230c3e1381fec71d82.png) ,
, ![\frac{d[B]}{dt}=k_1[A]](http://upload.wikimedia.org/math/7/0/c/70c1dea62ee39ded884e987e5c9aedf3.png) and
and ![\frac{d[C]}{dt}=k_2[A]](http://upload.wikimedia.org/math/a/0/b/a0b010b90ca7de616420b99117f81369.png)
The integrated rate equations are then ![\ [A] = [A]_0 e^{-(k_1+k_2)t}](http://upload.wikimedia.org/math/5/f/f/5fff2656e4c0d91000034a78835cd799.png) ;
; ![[B] = \frac{k_1}{k_1+k_2}[A]_0 (1-e^{-(k_1+k_2)t})](http://upload.wikimedia.org/math/9/8/f/98f477373e35c5723c85bc5629c32569.png) and
and ![[C] = \frac{k_2}{k_1+k_2}[A]_0 (1-e^{-(k_1+k_2)t})](http://upload.wikimedia.org/math/0/9/5/0953b7867deff6def848ed0b236d7709.png) .
.
One important relationship in this case is ![\frac{[B]}{[C]}=\frac{k_1}{k_2}](http://upload.wikimedia.org/math/a/d/4/ad4a86d319ac3e8f781d1c8e075422ae.png)
- One first order and one second order reaction:[4]
This can be the case when studying a bimolecular reaction and a simultaneous hydrolysis (which can be treated as pseudo order one) takes place: the hydrolysis complicates the study of the reaction kinetics, because some reactant is being "spent" in a parallel reaction. For example A reacts with R to give our product C, but meanwhile the hydrolysis reaction takes away an amount of A to give B, a byproduct: 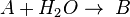 and
and 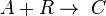 . The rate equations are:
. The rate equations are: ![\frac{d[B]}{dt}=k_1[A][H_2O]=k_1'[A]](http://upload.wikimedia.org/math/c/b/e/cbef138ce847e5fa0a11569b9739d693.png) and
and ![\frac{d[C]}{dt}=k_2[A][R]](http://upload.wikimedia.org/math/c/1/4/c146186b77712d9ace92327a4e91dd4b.png) . Where k1' is the pseudo first order constant.
. Where k1' is the pseudo first order constant.
The integrated rate equation for the main product [C] is ![[C]=[R]_0 \left [ 1-e^{-\frac{k_2}{k_1'}[A]_0(1-e^{-k_1't})} \right ]](http://upload.wikimedia.org/math/2/e/4/2e4456d93007140e9859124322dabbc6.png) , which is equivalent to
, which is equivalent to ![ln \frac{[R]_0}{[R]_0-[C]}=\frac{k_2[A]_0}{k_1'}(1-e^{-k_1't})](http://upload.wikimedia.org/math/d/f/6/df6c7da387064a2808460356cedee5ac.png) . Concentration of B is related to that of C through
. Concentration of B is related to that of C through ![[B]=-\frac{k_1'}{k_2} ln \left ( 1 - \frac{[C]}{[R]_0} \right )](http://upload.wikimedia.org/math/e/1/a/e1a1502f77a0da87232864d40a484f38.png)
The integrated equations were analytically obtained but during the process it was assumed that ![[A]_0-[C]\approx \;[A]_0](http://upload.wikimedia.org/math/8/4/b/84b23426a8836de817b0afce4d68e703.png) therefeore, previous equation for [C] can only be used for low concentrations of [C] compared to [A]0
therefeore, previous equation for [C] can only be used for low concentrations of [C] compared to [A]0

![r\; =\; k\left( T \right)\left[ A \right]^{n}\left[ B \right]^{m}](http://upload.wikimedia.org/math/3/0/a/30ad183024ad3fa780d7d362ba9f0e2a.png)

![r = -\frac{d[A]}{dt}=k](http://upload.wikimedia.org/math/4/2/6/426104668a2d1fe61fe4dbbb190d75f7.png)
![\ [A] = -kt + [A]_0](http://upload.wikimedia.org/math/d/e/f/defb5dedf555a3eedbdfd4a969be1597.png)
![\ r = k[A]](http://upload.wikimedia.org/math/3/3/9/339a09d222733a1a5ea96fe2fb39fab8.png)
![-\frac{1}{a}\frac{d[A]}{dt} = k[A]](http://upload.wikimedia.org/math/b/4/d/b4d8989af7a7c3f5de1d4251956742d4.png)
![\ \ln{[A]} = -akt + \ln{[A]_0}](http://upload.wikimedia.org/math/b/4/9/b4935c19a0f84fbb56fbe9b638324306.png)



![\frac{[A]}{[B]} = \frac{[A]_0}{[B]_0} e^{([A]_0 - [B]_0)kt}](http://upload.wikimedia.org/math/0/2/6/0261d2c287f23e6971bba8e5cdf68b22.png)
![r = {k_1 [A]^s[B]^t} - {k_2 [X]^u[Y]^v}\,](http://upload.wikimedia.org/math/5/7/f/57fac86d71637ea80bcdf8bad7a84b43.png)
![{k_1 [A]^s[B]^t = k_2 [X]^u[Y]^v}\,](http://upload.wikimedia.org/math/0/f/4/0f490754c2af17cdc6442dd2792ec008.png)
![K = \frac{[X]^u[Y]^v}{[A]^s[B]^t} = \frac{k_1}{k_2}](http://upload.wikimedia.org/math/a/0/9/a097aa22a4f852d3180cca18a45e2a04.png)

![K \ \stackrel{\mathrm{def}}{=}\ \frac{k_{f}}{k_{b}} = \frac{\left[B\right]_e} {\left[A\right]_e}](http://upload.wikimedia.org/math/0/5/b/05b33bde8d504ac56641d440c1e39e92.png)
![\ [A]_e = [A]_0 - x](http://upload.wikimedia.org/math/0/8/2/08214f2873c805d248b13e488c22856c.png)
![\ [B]_e = x](http://upload.wikimedia.org/math/0/a/4/0a46fb91b7f8e3cea51c86be61592d0c.png)
![\ [B]_e = x = \frac{k_{f}}{k_f+k_r}[A]_0](http://upload.wikimedia.org/math/b/7/f/b7f856c50659d902b04c6120a6b8e6f4.png)
![\ [A]_e = [A]_0 - x = \frac{k_{r}}{k_f+k_r}[A]_0](http://upload.wikimedia.org/math/a/8/d/a8d693dfc7cdec8572f9a5333498d875.png)
![\ \frac{dx}{dt} = \frac{k_f[A]_0}{x_e} (x_e - x)](http://upload.wikimedia.org/math/8/6/0/86088ab72ea98fc027158d36f533563d.png)
![\ ln(\frac{[A]_0 - [A]_e}{[A_t]-[A]_e}) = (k_f + k_r)t](http://upload.wikimedia.org/math/6/c/4/6c485a46a31086024e8c183cfabec06d.png)