Nuclide
From Wikipedia, the free encyclopedia
A nuclide (from lat.: nucleus) is a nuclear species which is characterized by the number of protons and neutrons that every atomic nucleus of this species contains.
For a short-hand designation of the nuclide, one writes the mass number (number of nucleons) in the upper left corner and the atomic number (number of protons) in the lower left corner of the chemical symbol: for example, 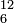 C for the most common isotope of carbon. In earlier years, the mass number was written in the upper right corner. The atomic number may also be omitted, since it is uniquely defined by the element symbol.
C for the most common isotope of carbon. In earlier years, the mass number was written in the upper right corner. The atomic number may also be omitted, since it is uniquely defined by the element symbol.
The various nuclides of a particular chemical element with equal proton number (atomic number), but different neutron numbers are called isotopes of this element. Before the term "nuclide" was internationally accepted (ca. 1950), the term "isotope" was also loosely used to describe a nuclear species, i.e., a nuclide. Nuclides with equal mass number but different atomic number are called isobars (isobar = equal in weight). Isotones are nuclides of equal neutron number but different proton numbers.
Nuclear isomers are atomic nuclei of a particular nuclide that have equal proton number and equal mass number, differ in energy content, and are long-lived (for example the two states of 99 Tc shown among the decay schemes). Unstable nuclides are radioactive and are called radionuclides.
| Designation | Characteristics | Example | Remarks |
|---|---|---|---|
| Isotopes | equal proton number | 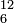 C , C ,  C C | |
| Isotones | equal neutron number |  C , C ,  N N | |
| Isobars | equal mass number |  N , N ,  O , O , 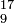 F F | see beta decay |
| Mirror nuclei | neutron and proton number exchanged |  H , H ,  He He | |
| Nuclear isomers | different energy states |  Tc Tc | long-lived or stable |
About 270 stable and about 70 unstable (radioactive) nuclei exist in nature. The natural radioisotopes are those whose half-lives T1/2 are at least as large as the age of the earth (4,6×109 years) (or they must have long-lived precursors). For example, the isotope 238U (T1/2 = 4,5×109 a) of uranium occurs in nature, but also the isotope 226Ra (T1/2 = 1602 a) of radium since this is a descendant of 238U. Many more than 1000 nuclides have been artificially produced.
The known nuclides are shown in charts of the nuclides (see Weblinks)
[edit] Weblinks
- Chart of the nuclides
- Details about the nuclides
- Periodic system with details of the nuclides
- Universal Nuclide Chart from Nucleonica
Category: Nuclear physics
