Limiting reagent
From Wikipedia, the free encyclopedia
In chemistry, the limiting reagent, or also called the "limiting reactant", is the chemical that determines how far the reaction will go before the chemical in question gets used up, causing the reaction to stop. The chemical of which there are fewer mols than the proportion requires is the limiting reagent.
[edit] Example
Consider the combustion of benzene:
1.5 mol C6H6 x 
This means that 11.25 mol O2 is required to react with 1.5 mol C6H6. Since only 7 mol O2 is present, the oxygen will be consumed before benzene. Therefore, O2 must be the limiting reagent.
This conclusion can be verified by comparing the mole ratio of O2 and C6H6 required by the balanced equation with the mole ratio actually present:
required:  =
= 
actual:  =
= 
Since the actual ratio is too small, O2 is the limiting reagent.
Consider a typical thermite reaction:
If 20.0 g of Fe2O3 are reacted with 8.00 g Al(s) in the thermite reaction, Which reactant is limiting?.
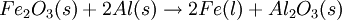
First, determine how many moles of Fe(l) can be produced from either reactant.
Moles produced of Fe from reactant Fe2O3



Moles produced of Fe from reactant Al
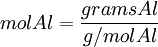

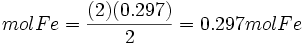
Because the moles Fe produced from Fe2O3(0.254mol) is less than the moles Fe produced from Al(0.297mol), Fe2O3 is the limiting reagent.
By looking at chemical equation for the thermite reaction, the limiting reagent can be found based on the ratio of moles of one reactant to another and the total atomic mass of the reactant compounds.
[edit] Reference
- Zumdahl, Steven S. Chemical Principals. 4th ed. New York: Houghton Mifflin Company, 2005. ISBN 0618372067.
