Ideal solution
From Wikipedia, the free encyclopedia
In chemistry, an ideal solution or ideal mixture is a solution in which the enthalpy of solution is zero [1]. The closer to zero the enthalpy of solution is, the more "ideal" the solution becomes. This is important in regard to colligative properties, where calculated, theoretical values become more accurate the more ideal the solution. Using a different definition, an ideal mixture is one in which the activity coefficients are equal to one.[2]
Ideality for solutions is analogous to ideality for gases, as described by the kinetic theory of matter in some ways. However there is a big difference as well. In ideal gases we assume that there are zero interactions between molecules, like or unlike. In the liquid such interactions are certainly not zero: they are strong. Instead we assume that they are the same between like or unlike neighbors.
Ideal solutions obey Raoult's Law over the entire composition range:
where
 is the equilibrium vapor pressure of the pure component
is the equilibrium vapor pressure of the pure component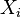 is the mole fraction of the component in solution
is the mole fraction of the component in solution
Using the Gibbs-Duhem relation we can show that if this expression holds true for one component over the whole range its must also hold for the other
According to Hildebrand, an ideal solution has a zero heat (or enthalpy) of mixing. That means that the Gibbs free energy is entirely determined by the entropy of mixing and this causes the G to have a single minimum at x= 0.5. Ideal solutions are therefore always completely miscible. It can also be shown that in the case of ideal solutions volumes remain strictly additive.
Note that Hildebrand's use of the term "ideal" as a good solvent differs from the theta temperature definition, which involves a thermodynamically poor solvent.
The total pressure is obtained by applying Dalton's law:
[edit] Chemical potential
| The factual accuracy of this section is disputed. Please see the relevant discussion on the talk page |
At chemical equilibrium the chemical potential of a solution is identical to that of the vapor for each component i:
The vapor phase is an ideal gas so:
and with Raoult's Law:
or
The terms in brackets are equal to the chemical potential of the pure liquid so
[edit] Non-ideality
For a solution to be ideal the interactions between like neighbors (UAB) and unlike neighborsUAA and UBB must be such that 2UAB=UAA+ UBB and the longer range interactions (than just with a neighbor) must be nil or at least the same.
This is hardly ever the case. Only if the molecules are almost identical chemically, e.g. 1-butanol and 2-butanol can we expect to approach this limit.
In many cases this is not so and we must find a way to incorporate non-ideality in the formulism. One way to do this is the use of a Margules function. Margules functions can have more than one parameter. Some systems with moderate non-ideality can be described with just one, they are called regular solutions.
In non-ideal cases volumes are no longer strictly additive and solubility does not have to be guaranteed over the whole composition range.







