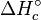Standard enthalpy change of combustion
From Wikipedia, the free encyclopedia
(Redirected from Enthalpy of combustion)
Standard enthalpy of Combustion is the change in enthalpy of the total reacting system (i.e. both products and reactants are considered the thermodynamic system) when one mole of a substance completely reacts with oxygen, and is observed at 298K and 1 atmospheric pressure (note: this is a temperature and pressure unlikely to facilitate combustion - adjustments are made to values after combustion is carried out normally). Typically, any enthalpy of combustion is measured in joules or kilojoules per mole of substance undergoing complete combustion.
Almost all reactions give a negative enthalpy change.
The standard enthalpy change of combustion is commonly denoted as  or
or