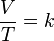Charles's law
From Wikipedia, the free encyclopedia
| Sections should be added to this article, to conform with Wikipedia's Manual of Style. Please discuss this issue on the talk page. |
In thermodynamics and physical chemistry, Charles' law is a gas law and specific instance of the ideal gas law, which states that:
- At constant pressure, the volume of a given mass of an ideal gas increases or decreases by the same factor as its temperature (in kelvin) increases or decreases.
The law was first published by Joseph Louis Gay-Lussac in 1802, but he referenced unpublished work by Jacques Charles from around 1787. This reference has led to the law being attributed to Charles. The relationship had been anticipated by the work of Guillaume Amontons in 1702.
The formula for the law is:
where:
- V is the volume of the gas
- T is the temperature of the gas (measured in Kelvins)
- k is a constant.
In other more thermodynamics-based definitions, the relationship between the fixed mass of a gas at constant pressure is inversely proportional to the temperature applied to the system, which can be further used by stipulating a system where α represents cubic expansivity of a gas, with θ representing the temperature measured of the system in Kelvins:
- V = Vo(1 + αθ)
To maintain the constant, k, during heating of a gas at fixed pressure, the volume must increase. Conversely, cooling the gas decreases the volume. The exact value of the constant need not be known to make use of the law in comparison between two volumes of gas at equal pressure:
 .
.
Basically, as the temperature increases the volume of the gas increases.