A neuromuscular junction is the synapse or junction of the axon terminal of a motor neuron with the motor end plate, as shown in Figures 1 and 2. The highly excitable region of muscle fiber plasma membrane is responsible for initiation of action potentials across the muscle's surface, ultimately causing the muscle to contract.
In vertebrates, the signal passes through the neuromuscular junction via the neurotransmitter acetylcholine.
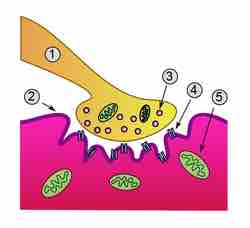
Figure 1. Detailed view of a neuromuscular junction
Detailed view of a neuromuscular junction: 1) Presynaptic terminal; 2) Sarcolemma; 3) Synaptic vesicle; 4) Nicotinic acetylcholine receptor; 5) Mitochondrion.
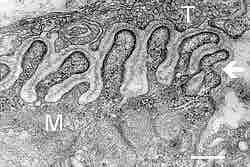
Figure 2. Neuromuscular junction
Electron micrograph showing a cross section through the neuromuscular junction. T is the axon terminal and M is the muscle fiber. The arrow shows junctional folds with basal lamina. Postsynaptic densities are visible on the tips between the folds. Scale is 0.3 µm.
Upon the arrival of an action potential at the presynaptic neuron terminal, voltage-dependent calcium channels open and Ca2+ ions flow from the extracellular fluid into the presynaptic neuron's cytosol. This influx of Ca2+ causes neurotransmitter-containing vesicles to dock and fuse to the presynaptic neuron's cell membrane, which results in the emptying of the vesicle's contents (acetylcholine) into the synaptic cleft; this process is known as exocytosis.
Acetylcholine diffuses into the synaptic cleft and binds to the nicotinic acetylcholine receptors located on the motor end plate.
These receptors open to allow sodium ions to flow in and potassium ions to flow out of the muscle's cytosol, producing a local depolarization of the motor end plate, known as an end-plate potential (EPP). This depolarization spreads across the surface of the muscle fiber and continues the excitation–contraction coupling to contract the muscle.
The action potential spreads through the muscle fiber's network of T-tubules, depolarizing the inner portion of the muscle fiber. The depolarization activates L-type, voltage-dependent calcium channels (dihydropyridine receptors) in the T-tubule membrane, which are in close proximity to calcium-release channels (ryanodine receptors) in the adjacent sarcoplasmic reticulum.
As intracellular calcium levels rise, the motor proteins responsible for the contractile response are able to interact, as shown in Figure 3, to form cross-bridges and undergo shortening.
Clinical Example: Myasthenia gravis is an autoimmune disorder in which circulating antibodies block the nicotinic acetylcholine receptors on the motor end plate of the neuromuscular junction. This blockage of acetylcholine receptors causes muscle weakness, often first exhibiting drooping eyelids and expanding to include overall muscle weakness and fatigue.
The affects of myasthenia gravis illustrate the importance of effective and functioning neuromuscular junctions for communication between neurons and muscles to allow contraction and relaxation of muscle fibers.
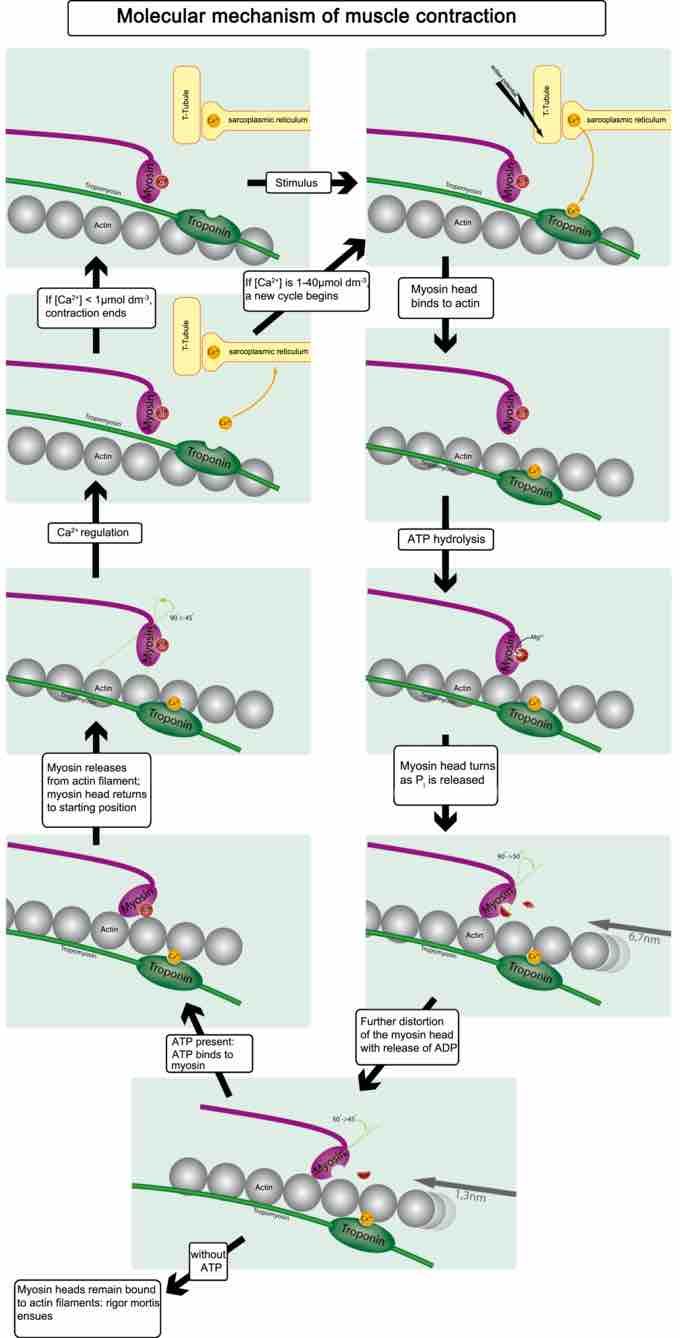
Figure 3. Muscle contraction and actin–myosin interactions
Skeletal muscle contracts following activation by an action potential. The binding of acetylcholine at the motor end plate leads to intracellular calcium release and interactions between myofibrils to elicit contraction.