Overview
The term relative humidity refers to how much water vapor is in the air compared with the maximum possible. At its maximum, denoted as saturation, the relative humidity is 100%, and evaporation is inhibited. The amount of water vapor the air can hold depends on its temperature. For example, relative humidity rises in the evening, as air temperature declines, sometimes reaching the dew point. At the dew point temperature, relative humidity is 100%, and fog may result from the condensation of water droplets if they are small enough to stay in suspension. Conversely, if one wished to dry something, it is more effective to blow hot air over it rather than cold air, because, among other things, hot air can hold more water vapor.
Evaporation
The capacity of air to hold water vapor is based on vapor pressure of water. The liquid and solid phases are continuously giving off vapor because some of the molecules have high enough speeds to enter the gas phase, a process called evaporation; see (a). For the molecules to evaporate, they must be located near the surface, be moving in the proper direction, and have sufficient kinetic energy to overcome liquid-phase intermolecular forces. When only a small proportion of the molecules meet these criteria, the rate of evaporation is low. Since the kinetic energy of a molecule is proportional to its temperature, evaporation proceeds more quickly at higher temperatures.
If a lid is placed over the container, as in (b), evaporation continues, increasing the pressure, until sufficient vapor has built up for condensation to balance evaporation. Then equilibrium has been achieved, and the vapor pressure is equal to the partial pressure of water in the container. Vapor pressure increases with temperature because molecular speeds are higher as temperature increases.
As the faster-moving molecules escape, the remaining molecules have lower average kinetic energy, and the temperature of the liquid decreases. This phenomenon is also called evaporative cooling. This is why evaporating sweat cools the human body. Evaporation also tends to proceed more quickly with higher flow rates between the gaseous and liquid phase and in liquids with higher vapor pressure. For example, laundry on a clothes line will dry (by evaporation) more rapidly on a windy day than on a still day.
Application for Boiling Water
Why does water boil at 100ºC? The vapor pressure of water at 100ºC is 1.01×105 Pa, or 1.00 atm. Thus, it can evaporate without limit at this temperature and pressure. But why does it form bubbles when it boils? This is because water ordinarily contains significant amounts of dissolved air and other impurities, which are observed as small bubbles of air in a glass of water. If a bubble starts out at the bottom of the container at 20ºC, it contains water vapor (about 2.30%). The pressure inside the bubble is fixed at 1.00 atm (we ignore the slight pressure exerted by the water around it). As the temperature rises, the amount of air in the bubble stays the same, but the water vapor increases; the bubble expands to keep the pressure at 1.00 atm. At 100ºC, water vapor enters the bubble continuously since the partial pressure of water is equal to 1.00 atm in equilibrium. It cannot reach this pressure, however, since the bubble also contains air and total pressure is 1.00 atm. The bubble grows in size and thereby increases the buoyant force. The bubble breaks away and rises rapidly to the surface, resulting in boiling. (See . )
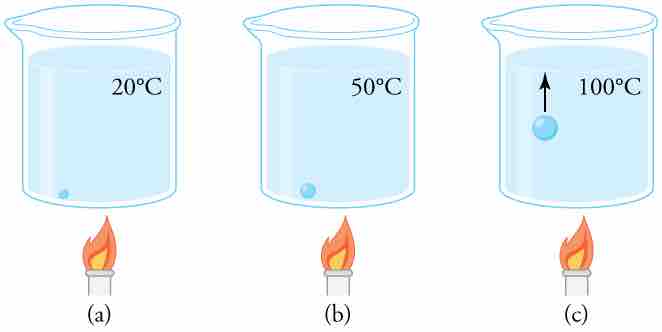
Close-up of the Boiling Process
(a) An air bubble in water starts out saturated with water vapor at 20ºC. (b) As the temperature rises, water vapor enters the bubble because its vapor pressure increases. The bubble expands to keep its pressure at 1.00 atm. (c) At 100ºC, water vapor enters the bubble continuously because water's vapor pressure exceeds its partial pressure in the bubble, which must be less than 1.00 atm. The bubble grows and rises to the surface.