The stability of an atom depends on the ratio of its protons to its neutrons, as well as on whether it contains a "magic number" of neutrons or protons that would represent closed and filled quantum shells. These quantum shells correspond to energy levels within the shell model of the nucleus. Filled shells, such as the filled shell of 50 protons in the element tin, confers unusual stability on the nuclide. Of the 254 known stable nuclides, only four have both an odd number of protons and an odd number of neutrons:
- hydrogen-2 (deuterium)
- lithium-6
- boron-10
- nitrogen-14
Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life greater than a billion years:
- potassium-40
- vanadium-50
- lanthanum-138
- tantalum-180m
Most odd-odd nuclei are highly unstable with respect to beta decay because the decay products are even-even and therefore more strongly bound, due to nuclear pairing effects.
An atom with an unstable nucleus, called a radionuclide, is characterized by excess energy available either for a newly created radiation particle within the nucleus or via internal conversion. During this process, the radionuclide is said to undergo radioactive decay. Radioactive decay results in the emission of gamma rays and/or subatomic particles such as alpha or beta particles, as shown in . These emissions constitute ionizing radiation. Radionuclides occur naturally but can also be produced artificially.
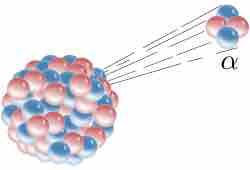
Alpha Decay
Alpha decay is one type of radioactive decay. An atomic nucleus emits an alpha particle and thereby transforms ("decays") into an atom with a mass number smaller by four and an atomic number smaller by two. Many other types of decay are possible.
All elements form a number of radionuclides, although the half-lives of many are so short that they are not observed in nature. Even the lightest element, hydrogen, has a well-known radioisotope: tritium. The heaviest elements (heavier than bismuth) exist only as radionuclides. For every chemical element, many radioisotopes that do not occur in nature (due to short half-lives or the lack of a natural production source) have been produced artificially.