Gamma radiation, also known as gamma rays and denoted as
Gamma decay accompanies other forms of decay, such as alpha and beta decay; gamma rays are produced after the other types of decay occur. When a nucleus emits an α or β particle, the daughter nucleus is usually left in an excited state. It can then move to a lower energy state by emitting a gamma ray, in much the same way that an atomic electron can jump to a lower energy state by emitting a photon. For example, cobalt-60 decays to excited nickel-60 by beta decay through emission of an electron of 0.31 MeV. Next, the excited nickel-60 drops down to the ground state by emitting two gamma rays in succession (1.17 MeV, then 1.33 MeV), as shown in . Emission of a gamma ray from an excited nuclear state typically requires only
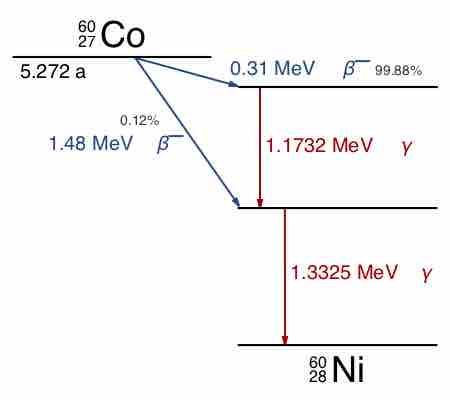
Cobalt-60 Decay Scheme
Path of decay of Co-60 to Ni-60. Excited levels for Ni-60 that drop to ground state via emission of gamma rays are indicated
In certain cases, the excited nuclear state following the emission of a beta particle may be more stable than average; in these cases it is termed a metastable excited state if its decay is 100 to 1000 times longer than the average