Mass Spectrometry
Mass spectrometry (MS) is the art of displaying the spectra (singular spectrum) of the masses of a sample of material. It is used for determining the elemental composition of a sample, and properties of the particles and molecules (the chemical structures of molecules, such as peptides and other chemical compounds).
Mass spectrometers, as diagramed in , separate compounds based on a property known as the mass-to-charge ratio. The sample to be identified is first ionized, and then passed through some form of magnetic field. Based on parameters, such as how long it takes the molecule to travel a certain distance or the amount of deflection caused by the field, a mass can be calculated for the ion.
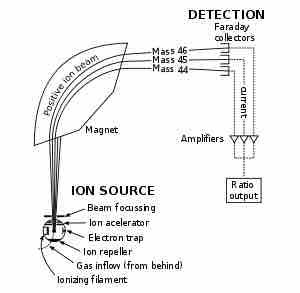
Schematic of Mass Spectrometer
Schematics of a simple mass spectrometer with sector type mass analyzer. This one is for the measurement of carbon dioxide isotope ratios as in the carbon-13urea breath test.
How it Works
First, the sample undergoes vaporization by intense heat. The gaseous components of the sample are each ionized (turned into ions) with the same quantity of charge. The ions are then grouped by applying a similar magnetic force to each. Since the acceleration of a charge is dependent on the mass and strength of the charge, a lighter mass-to-charge ratio will not travel as far as a high mass-to-charge ratio, allowing for comparison of the physical properties of different particles. The groupings are detected by some quantitative signal from of ongoing probing. The detector differentiates the ions based on how far they curve in the magnetic field. The signal is processed into the spectra (singular of spectrum) of the masses of the particles of that sample. The elements or molecules are uniquely identified by correlating known masses by the identified masses.