A laser is a device that emits monochromatic light (electromagnetic radiation). It does so through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation. Laser is distinct from other light sources for its high degree of spatial and temporal coherence, which means that laser outputs a narrow beam that maintains its temporal-phase relationship.
Principles of laser operation are largely based on quantum mechanics. (One exception would be free-electron lasers, whose operation can be explained solely by classical electrodynamics. ) When an electron is excited from a lower-energy to a higher-energy level, it will not stay that way forever. An electron in an excited state may decay to an unoccupied lower-energy state according to a particular time constant characterizing that transition. When such an electron decays without external influence, it emits a photon; this process is called "spontaneous emission. " The phase associated with the emitted photon is random. A material with many atoms in an excited state may thus result in radiation that is very monochromatic, but the individual photons would have no common phase relationship and would emanate in random directions. This is the mechanism of fluorescence and thermal emission.
However, an external photon at a frequency associated with the atomic transition can affect the quantum mechanical state of the atom . As the incident photon passes by, the rate of transitions of the excited atom can be significantly enhanced beyond that due to spontaneous emission. This "induced" decay process is called stimulated emission. In stimulated emission, the decaying atom produces an identical "copy" of the incoming photon. Therefore, after the atom decays, we have two identical outgoing photons. Since there was only one incoming photon, we amplified the intensity of light by a factor of 2!
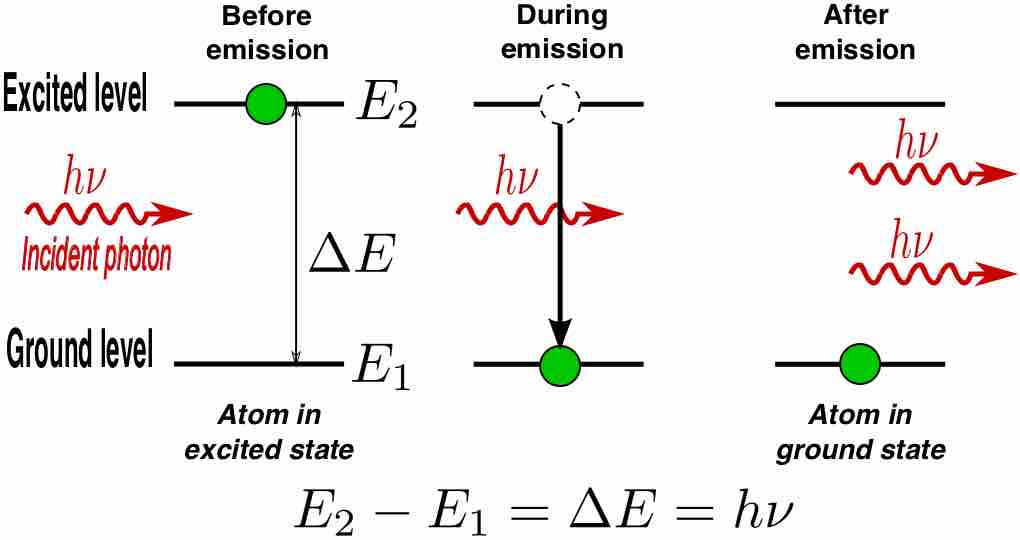
Stimulated Photon Emission
In stimulated emission process, a photon (with a frequency equal to the atomic transition) encounters an excited atom, and a new photon identical to the incoming photon is produced. The result is an atom in the ground state with two outgoing photons.