Concept
Version 7
Created by Boundless
Latent Heat
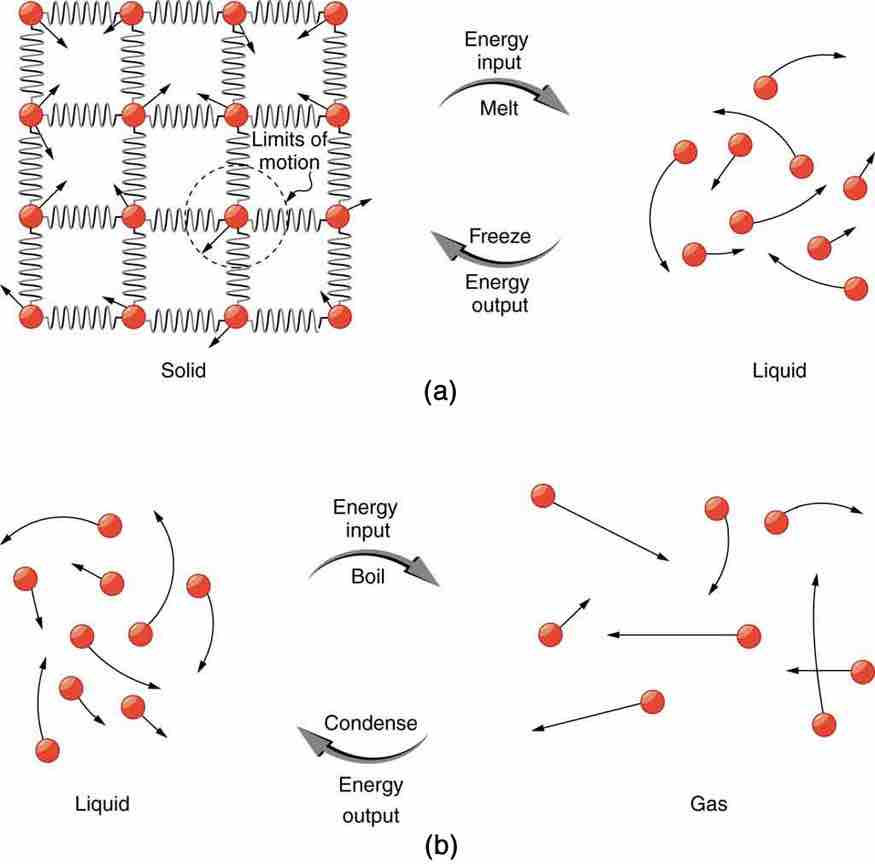
Phase Transitions
(a) Energy is required to partially overcome the attractive forces between molecules in a solid to form a liquid. That same energy must be removed for freezing to take place. (b) Molecules are separated by large distances when going from liquid to vapor, requiring significant energy to overcome molecular attraction. The same energy must be removed for condensation to take place. There is no temperature change until a phase change is complete.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. October 15, 2012."
http://cnx.org/content/m42225/latest/?collection=col11406/1.7
OpenStax CNX
CC BY 3.0.