Concept
Version 7
Created by Boundless
Latent Heat
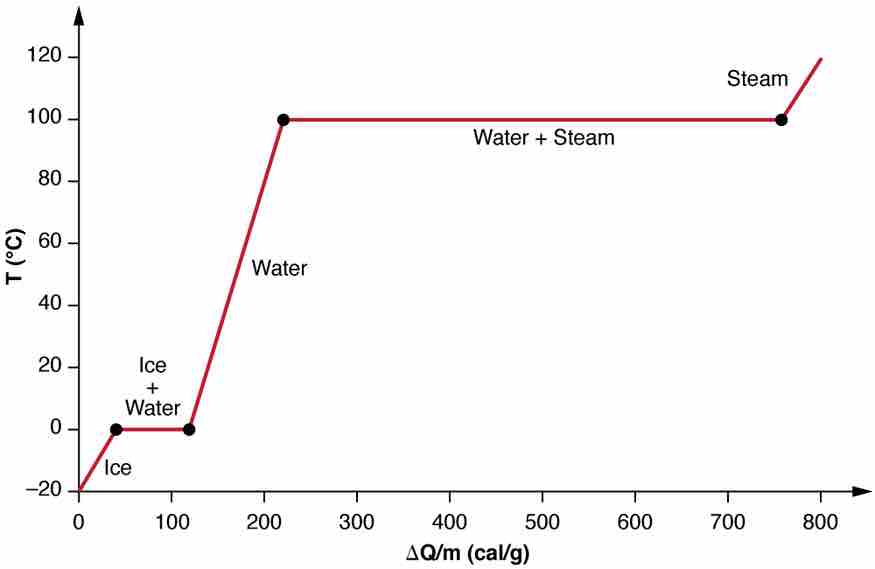
Heating and Phase Changes of Water
A graph of temperature versus energy added. The system is constructed so that no vapor evaporates while ice warms to become liquid water, and so that, when vaporization occurs, the vapor remains in of the system. The long stretches of constant temperature values at 0ºC and 100ºC reflect the large latent heat of melting and vaporization, respectively.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, College Physics. October 15, 2012."
http://cnx.org/content/m42225/latest/?collection=col11406/1.7
OpenStax CNX
CC BY 3.0.